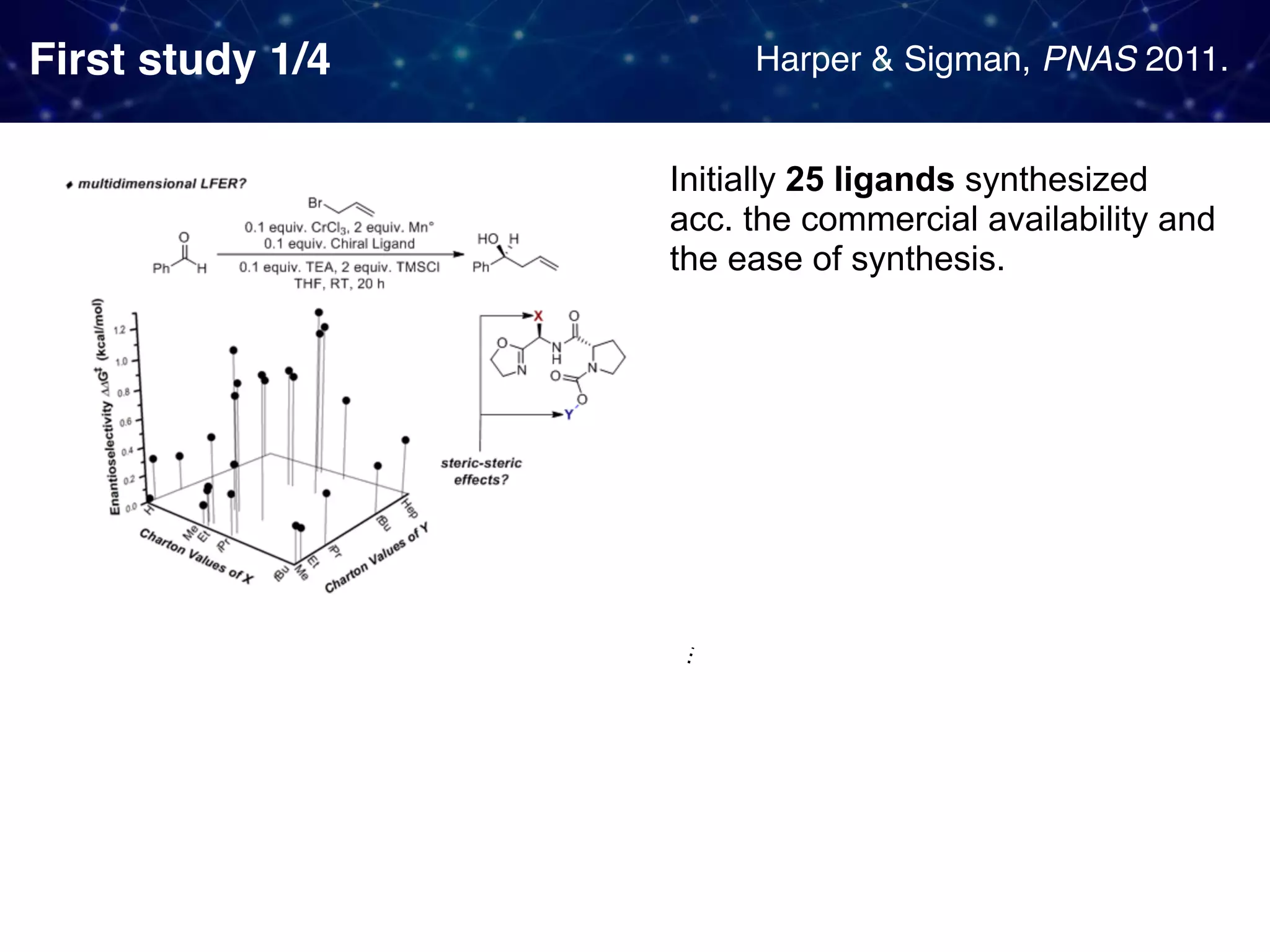
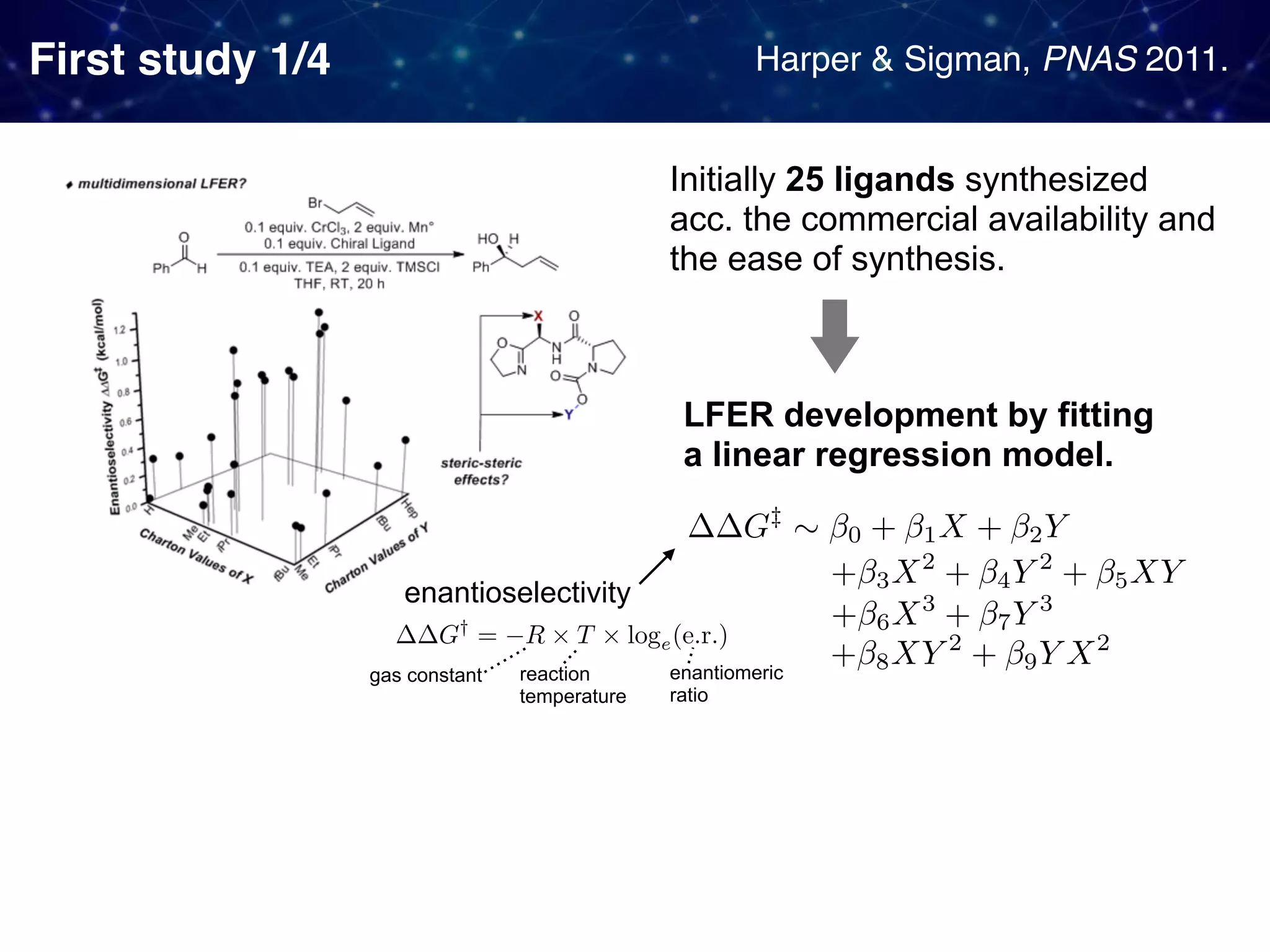
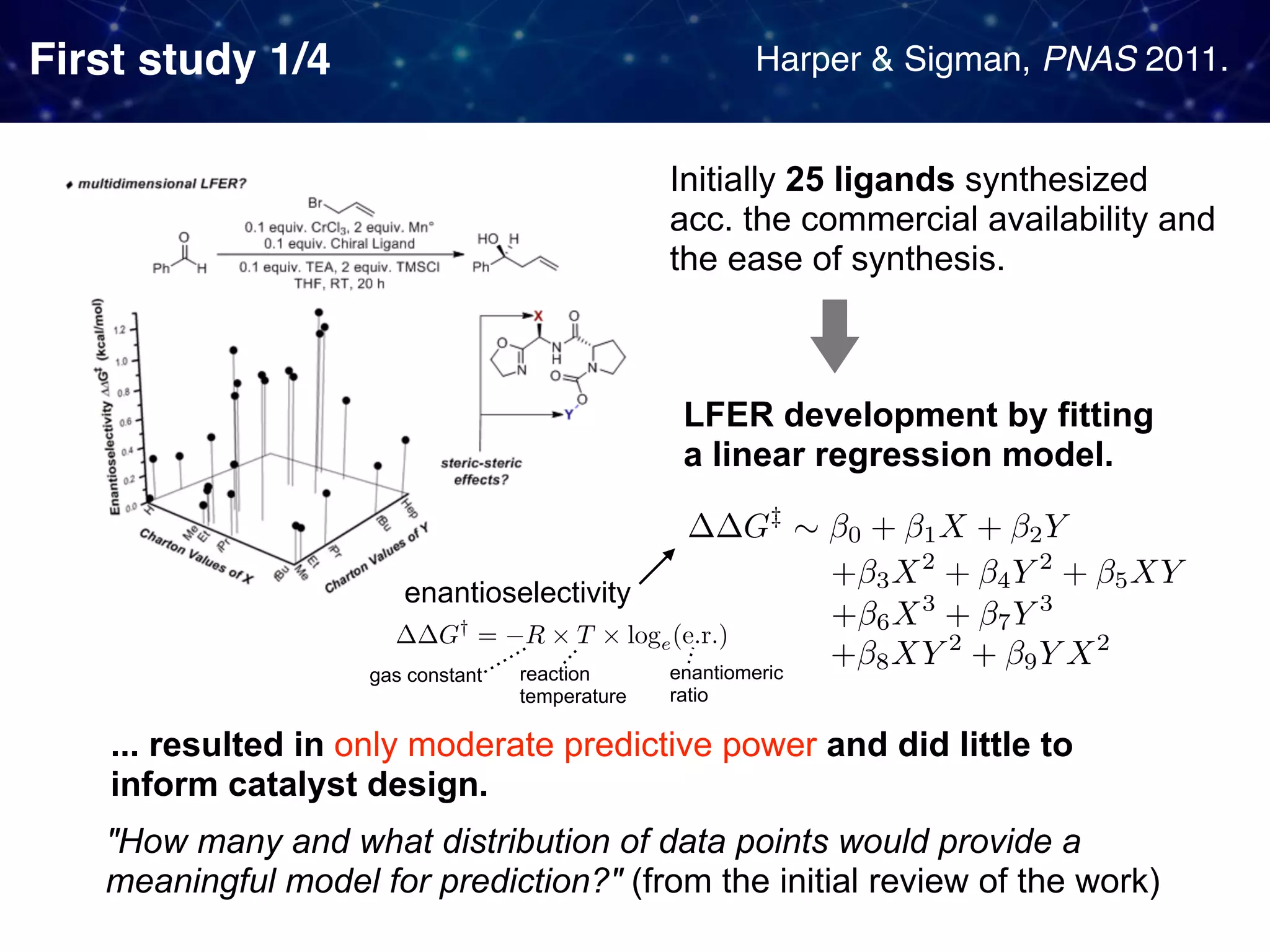
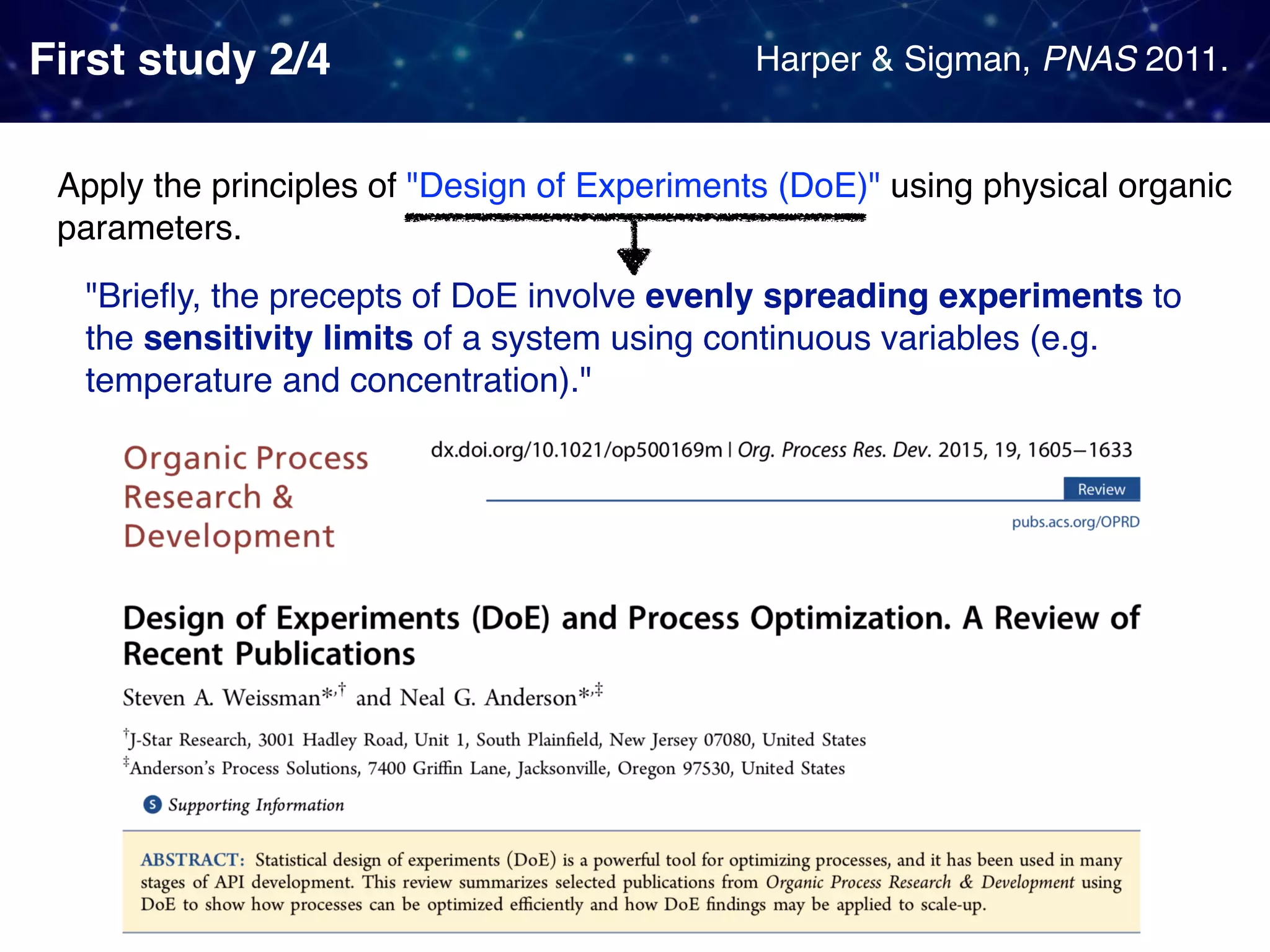
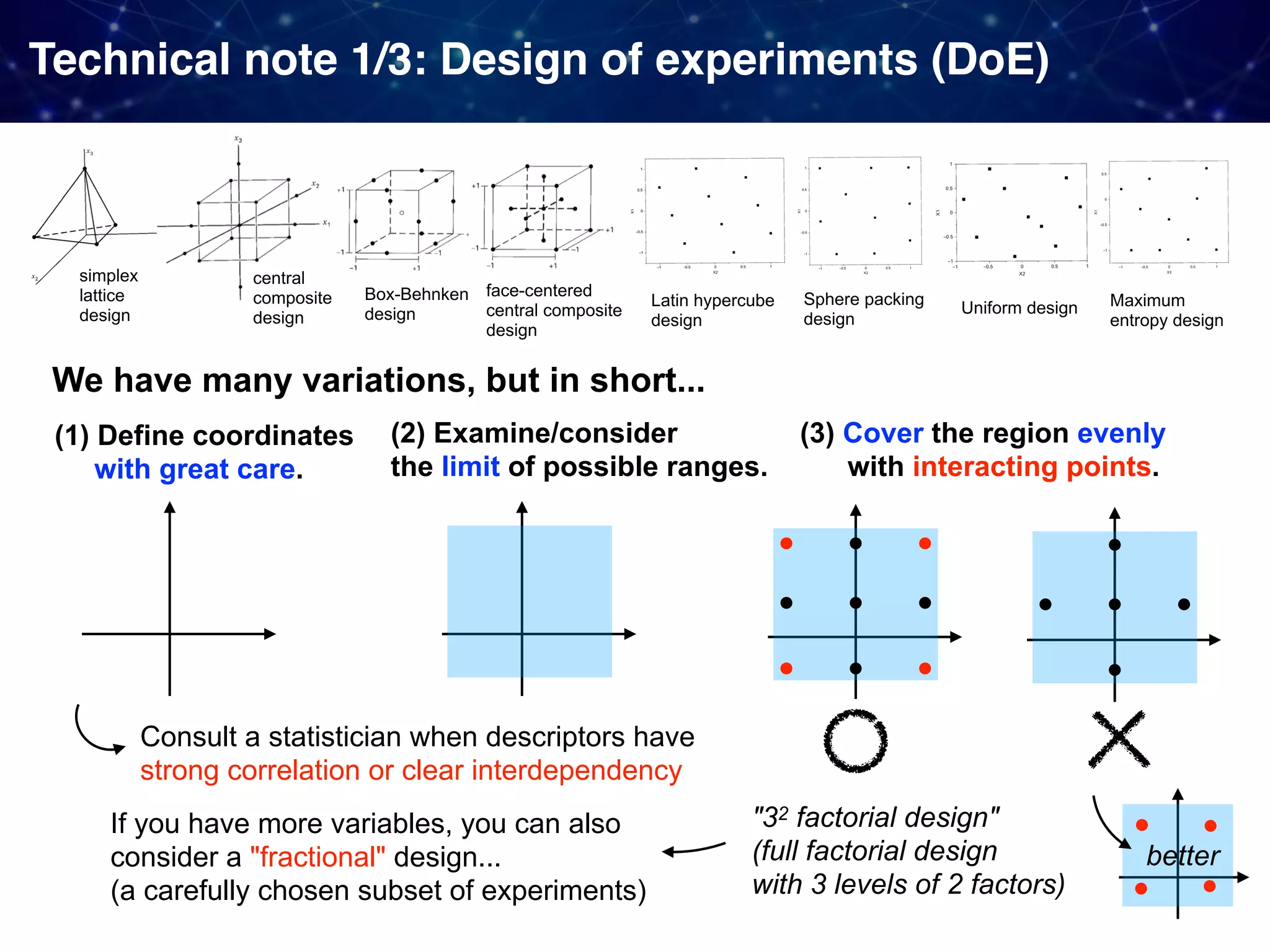
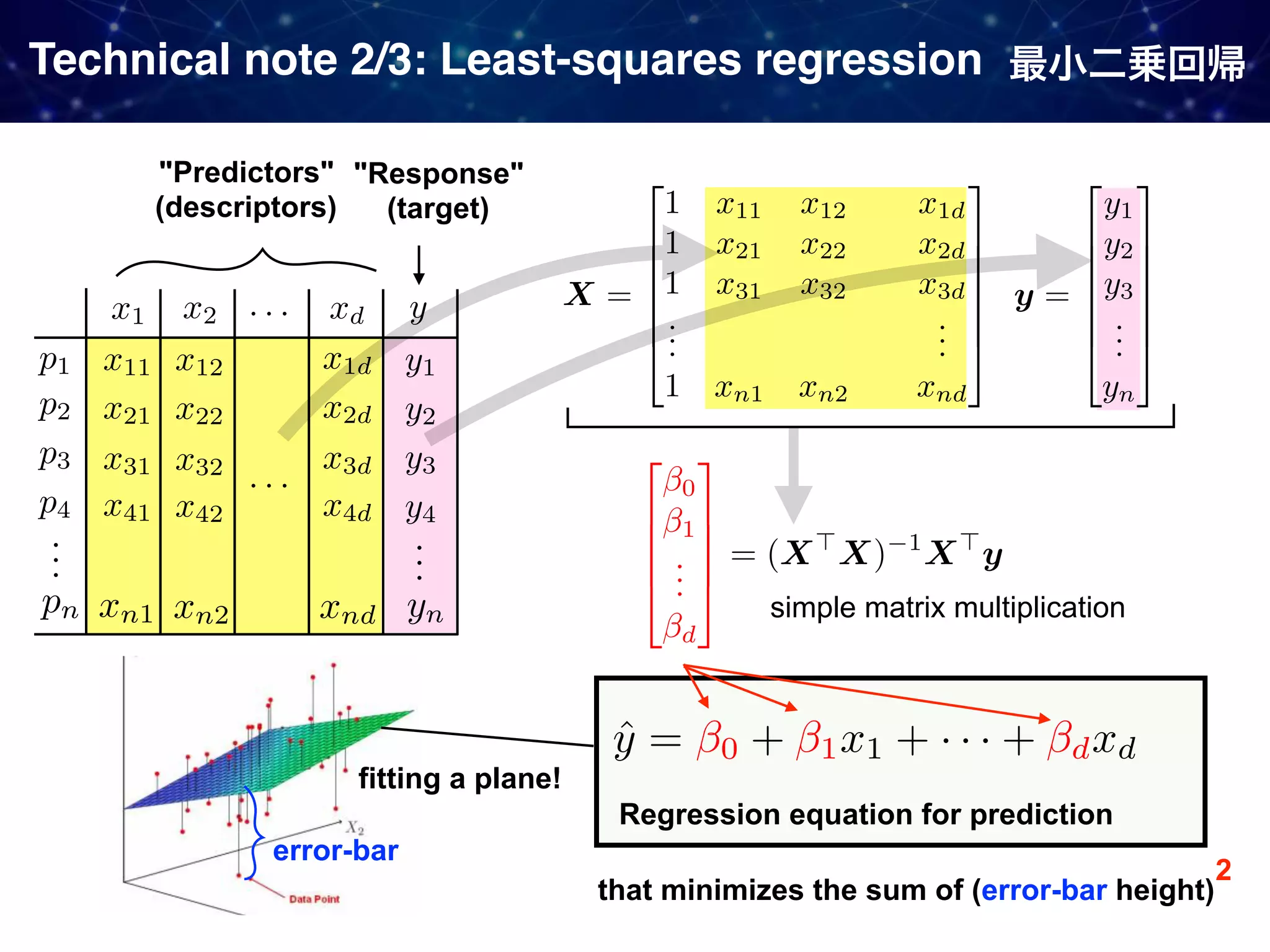
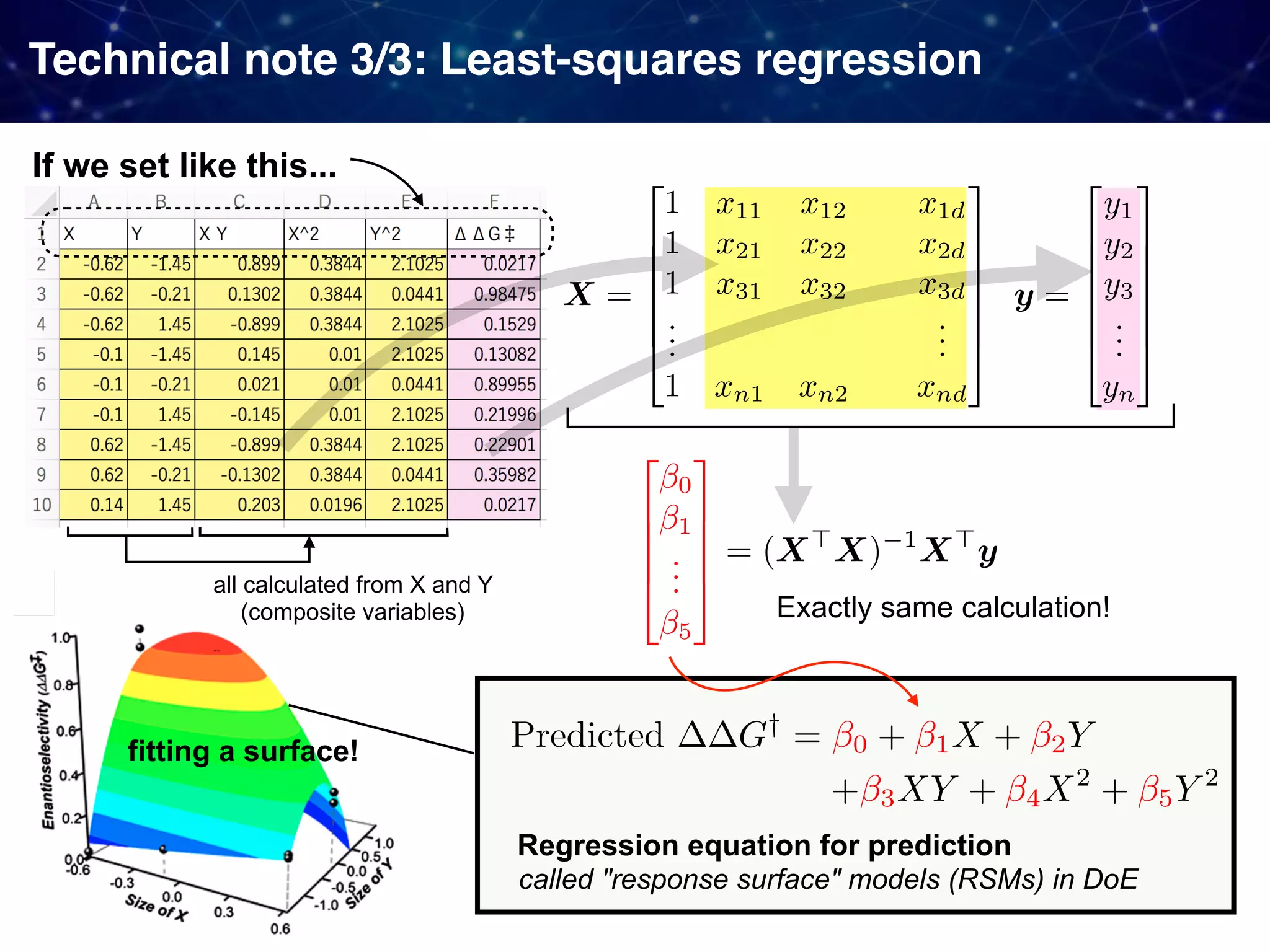
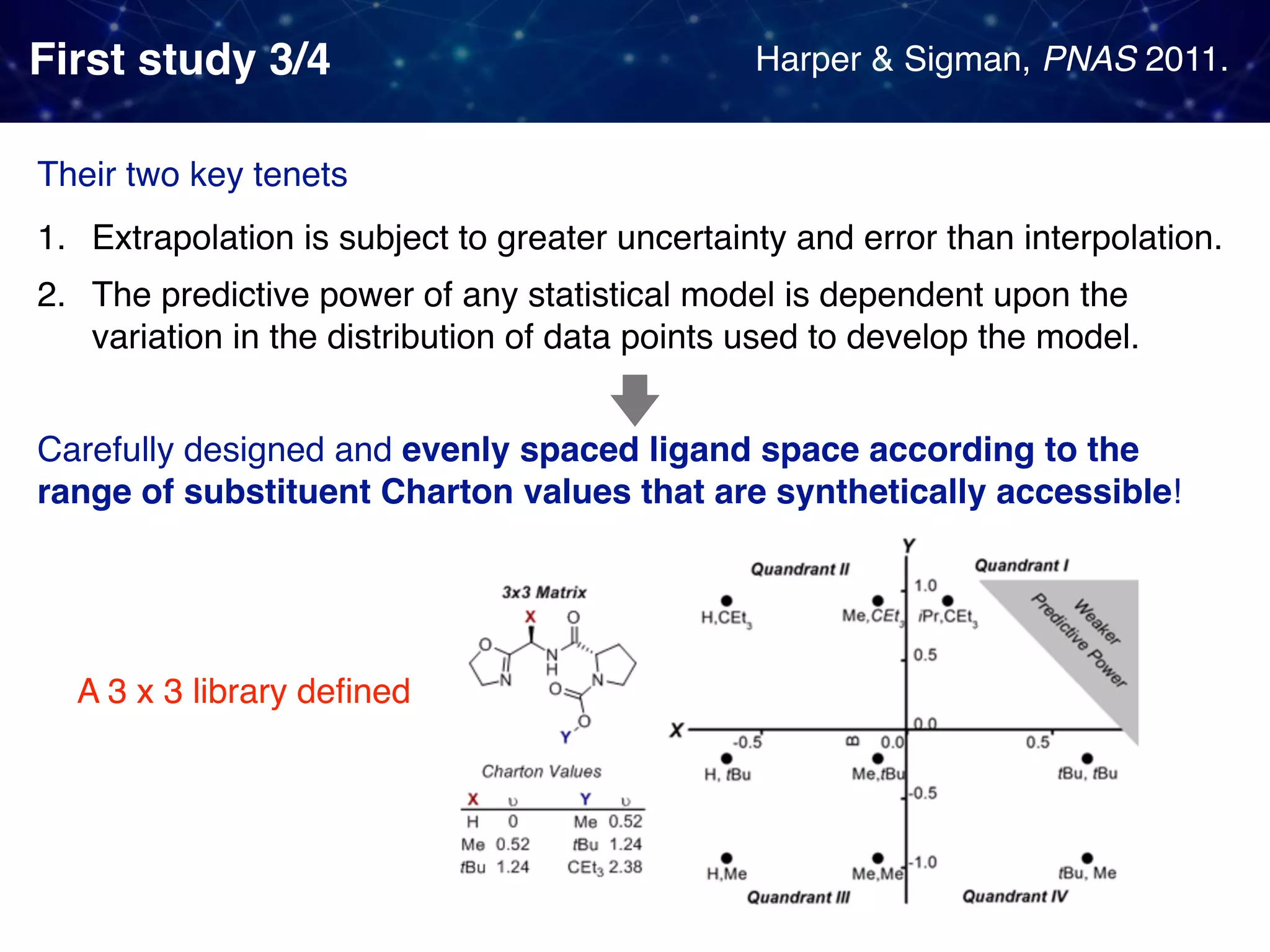
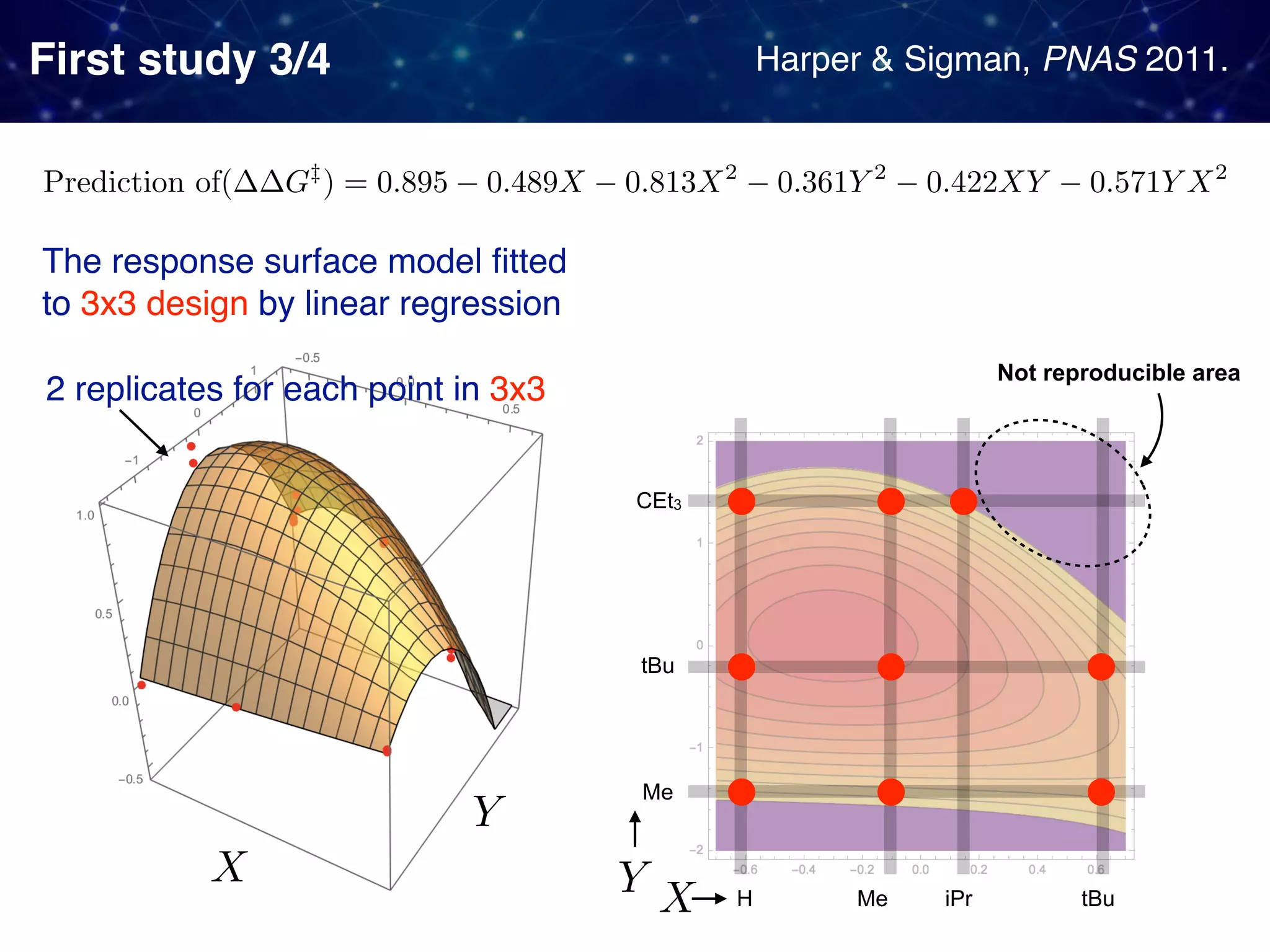
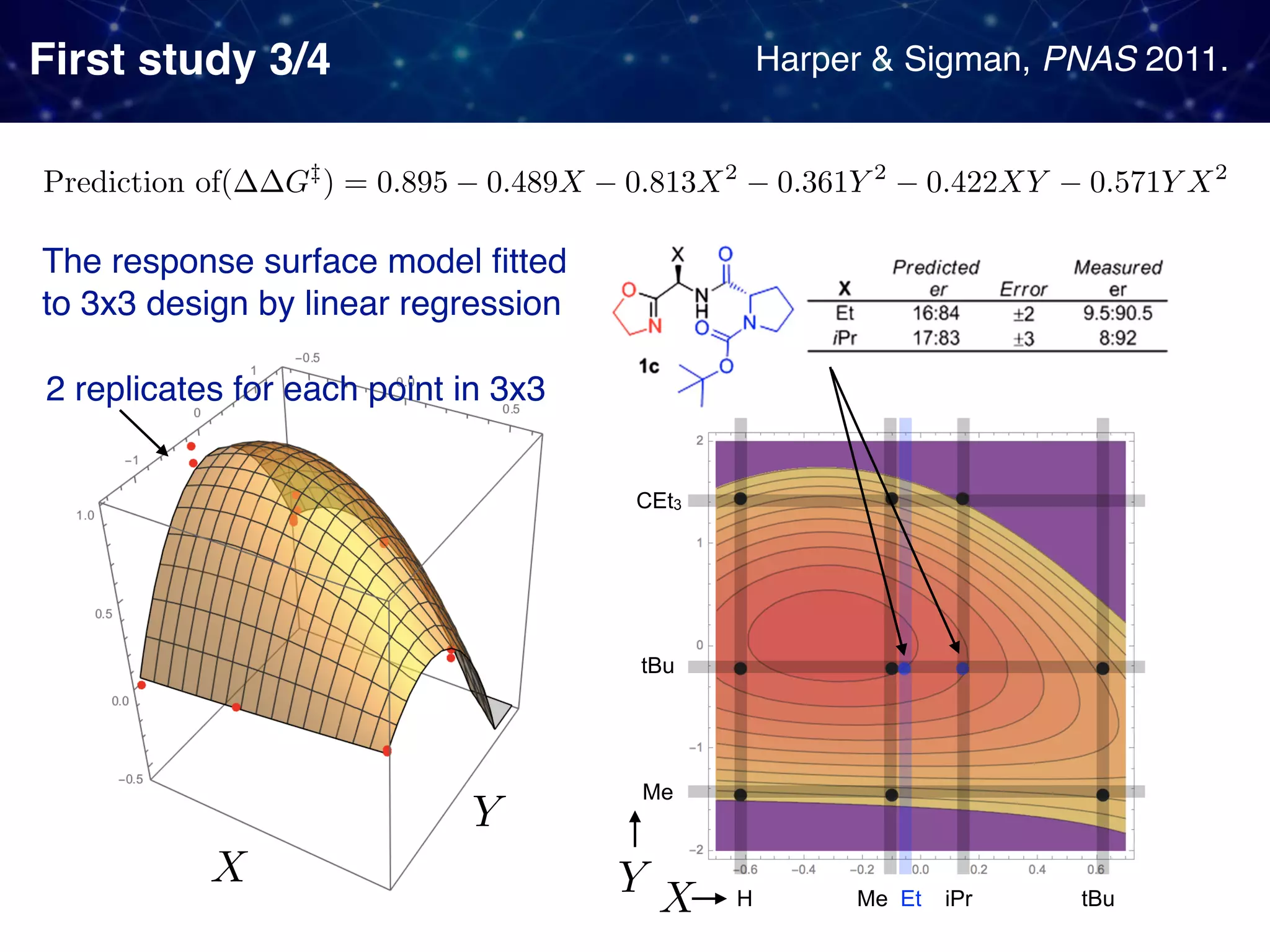
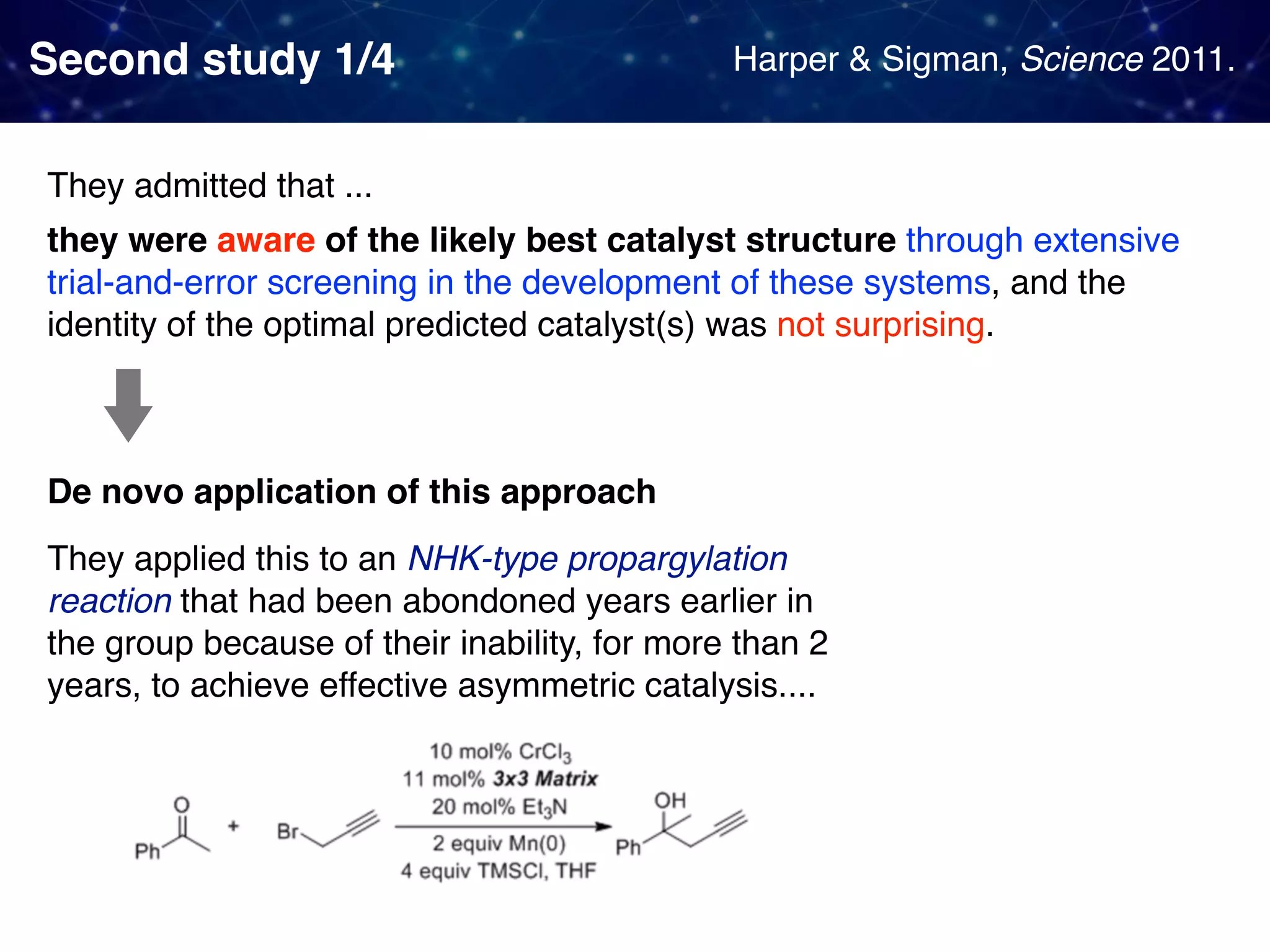
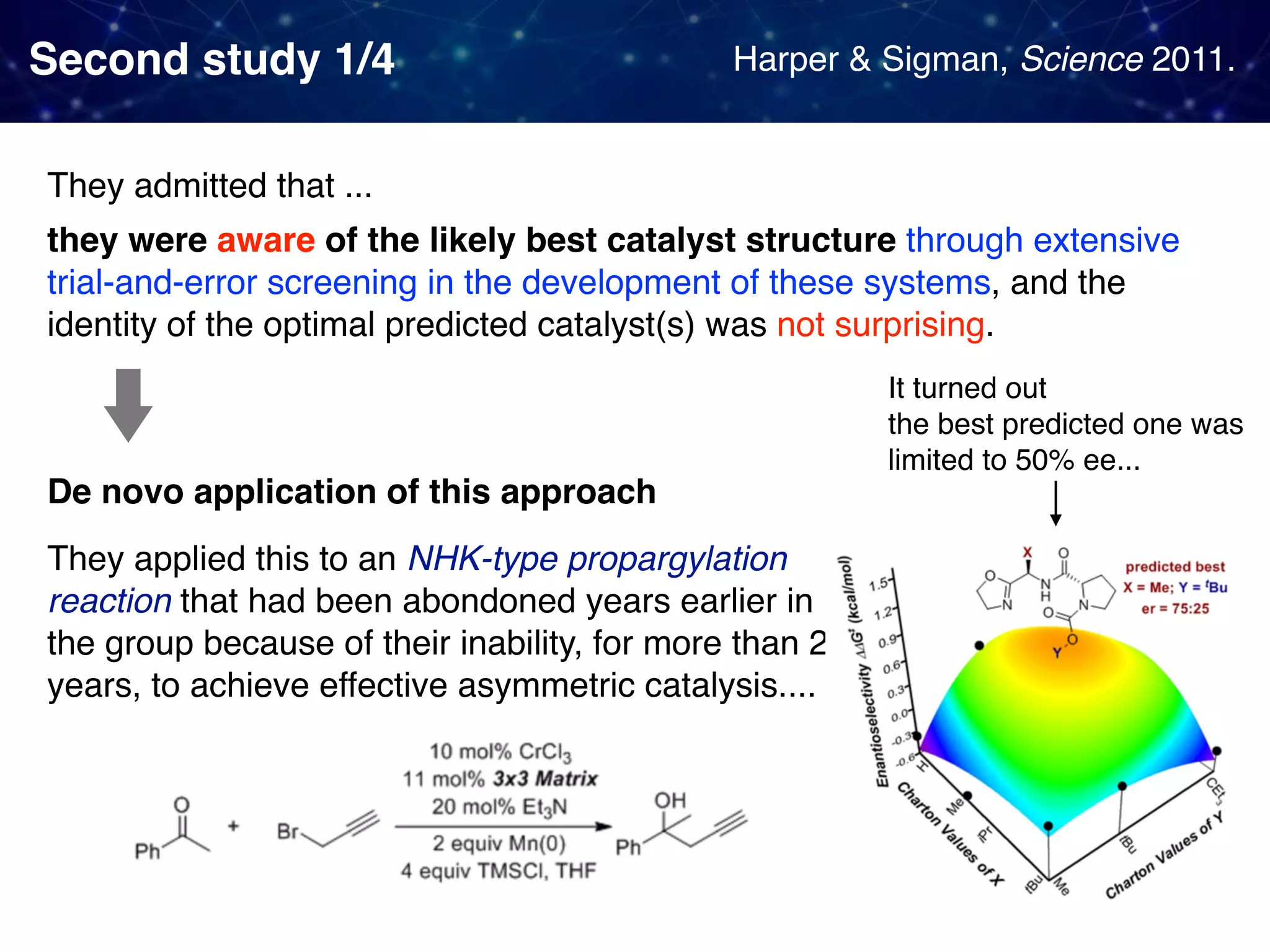
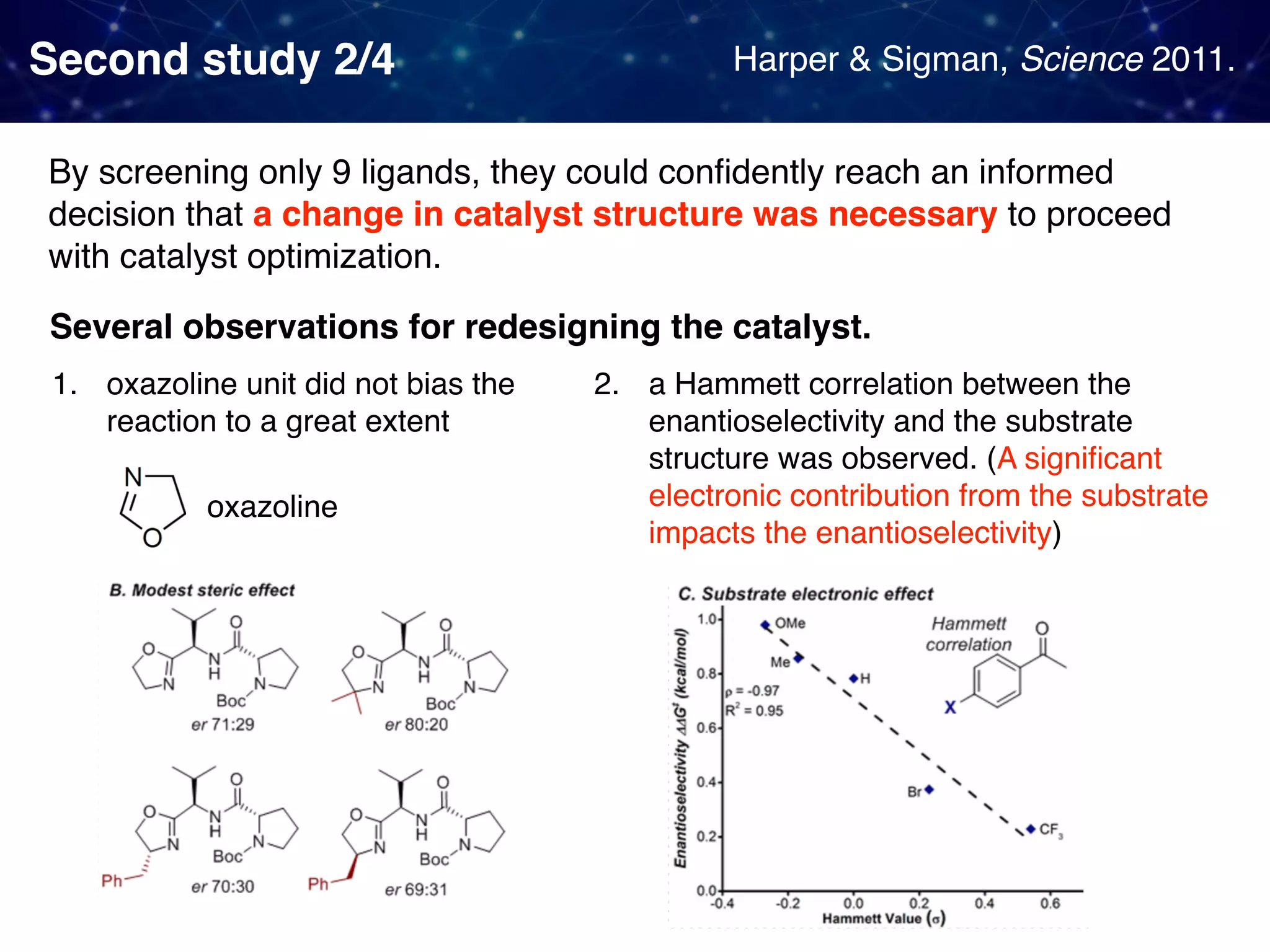
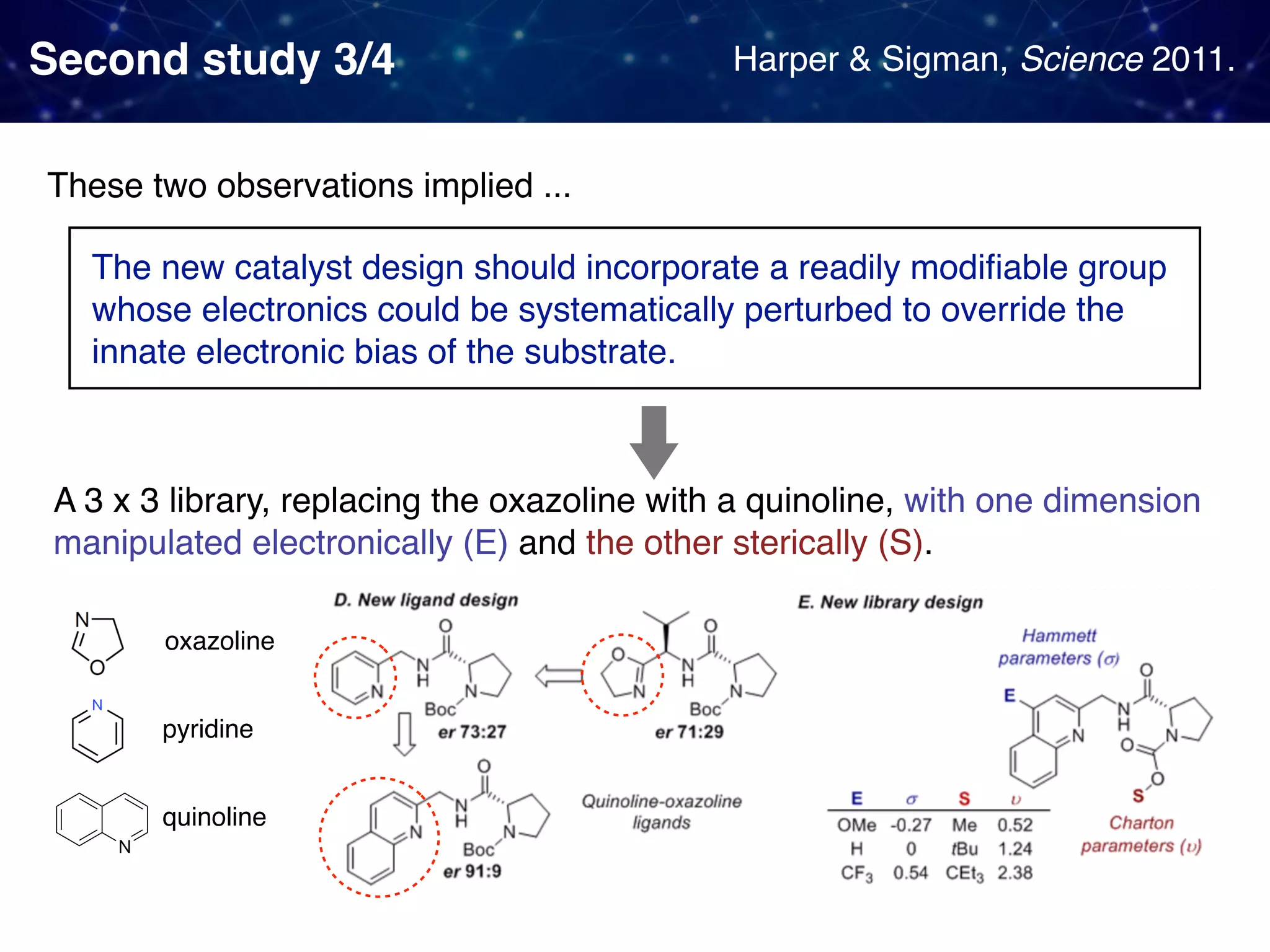
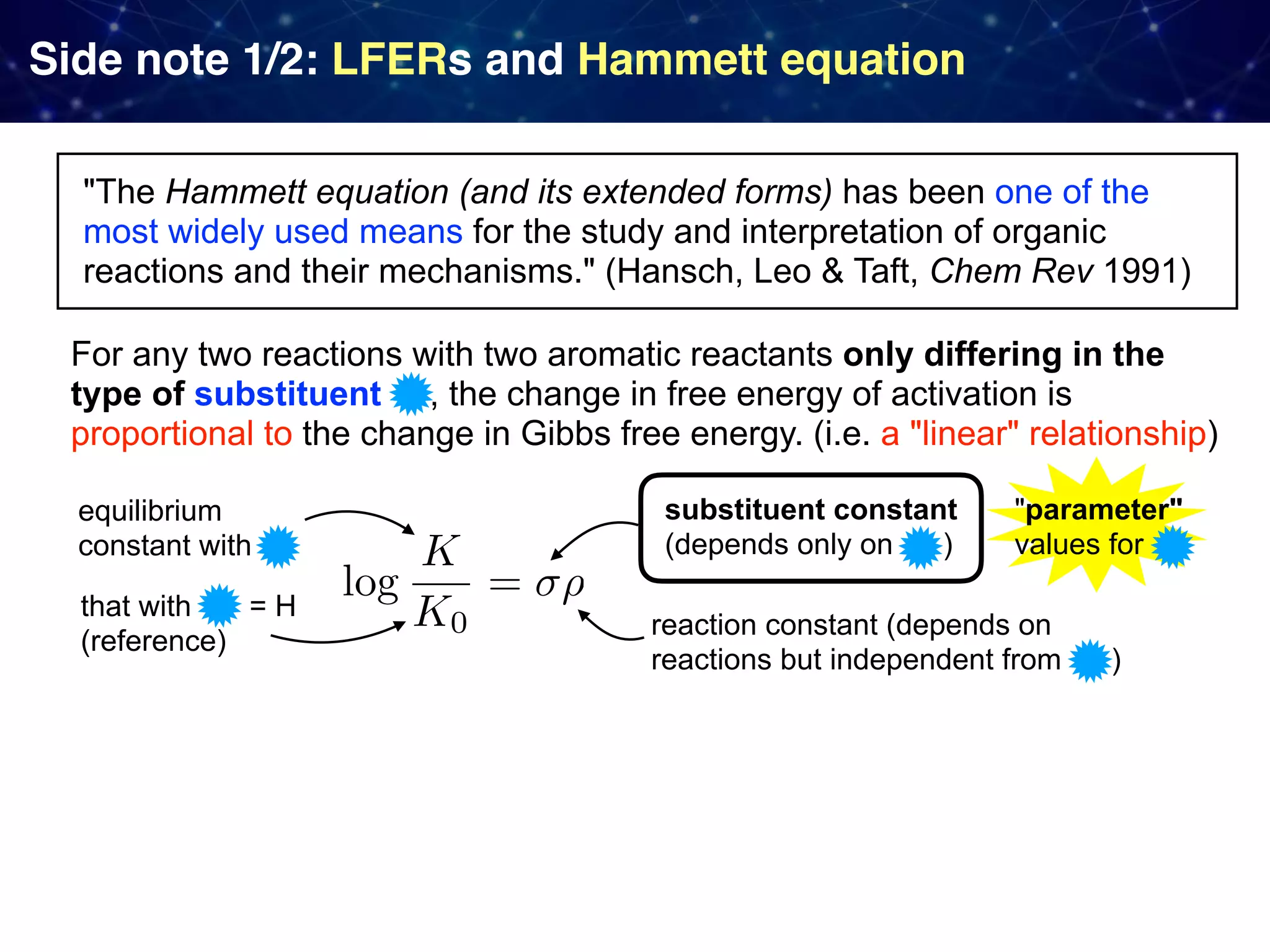
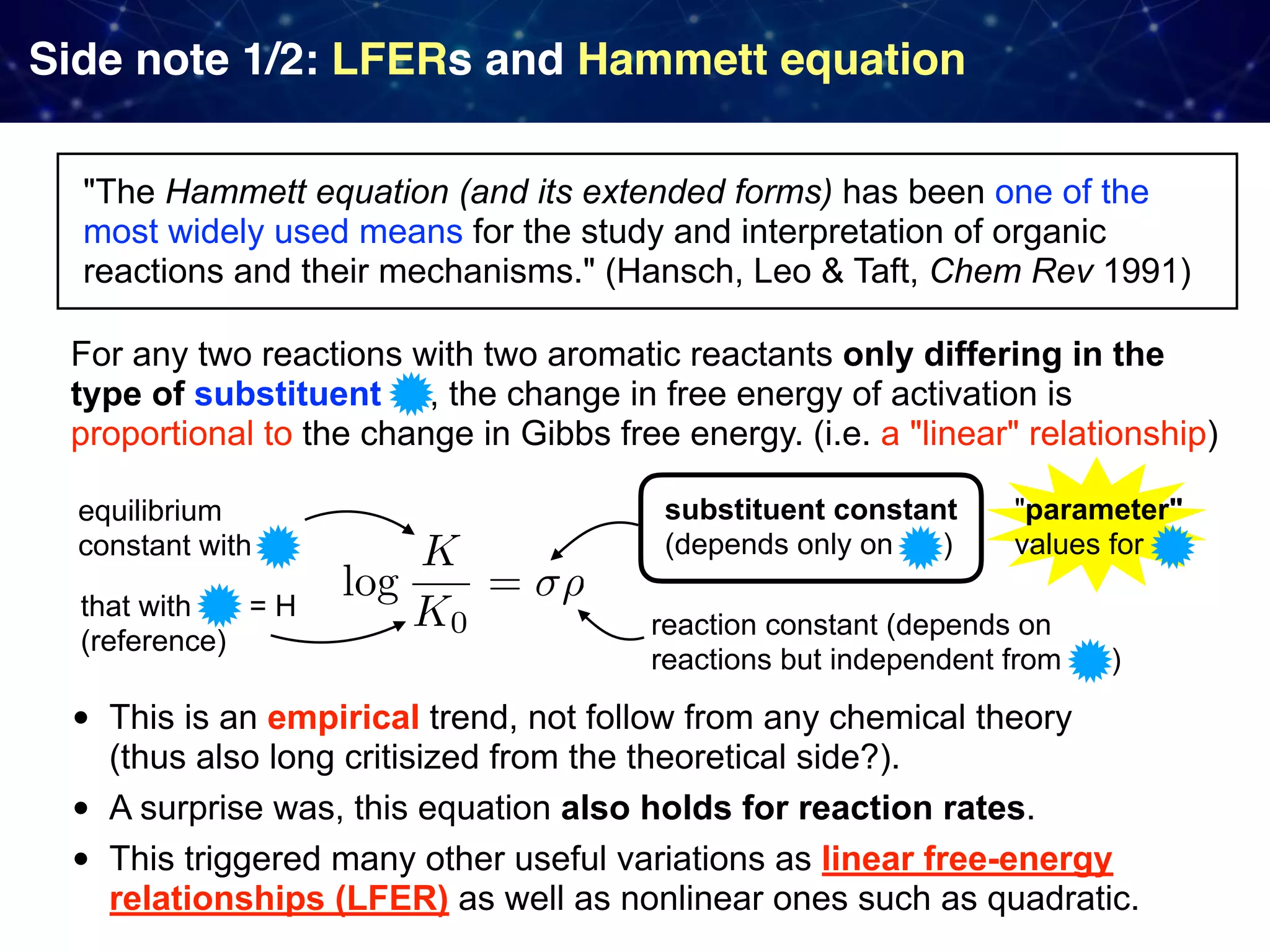
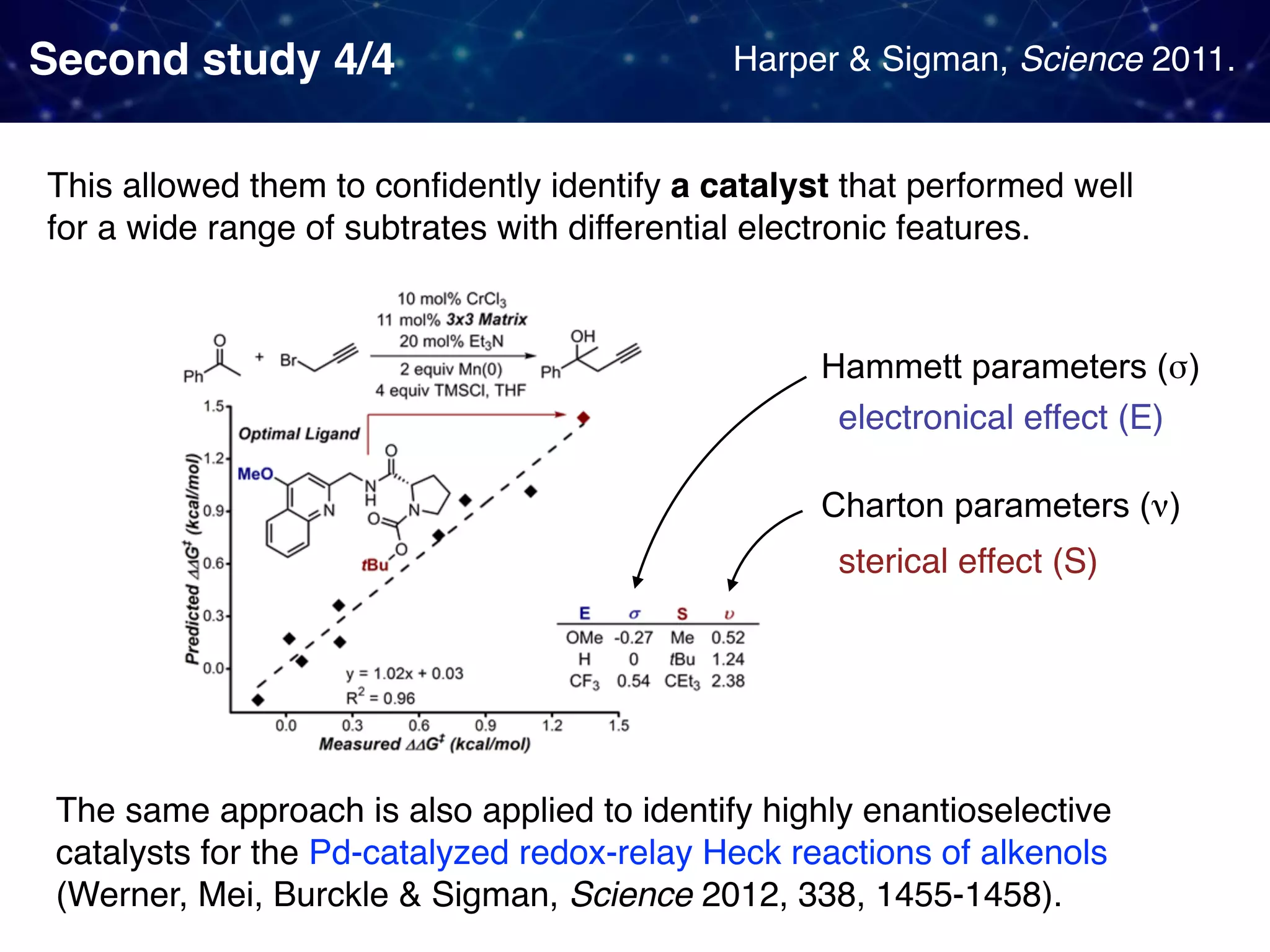
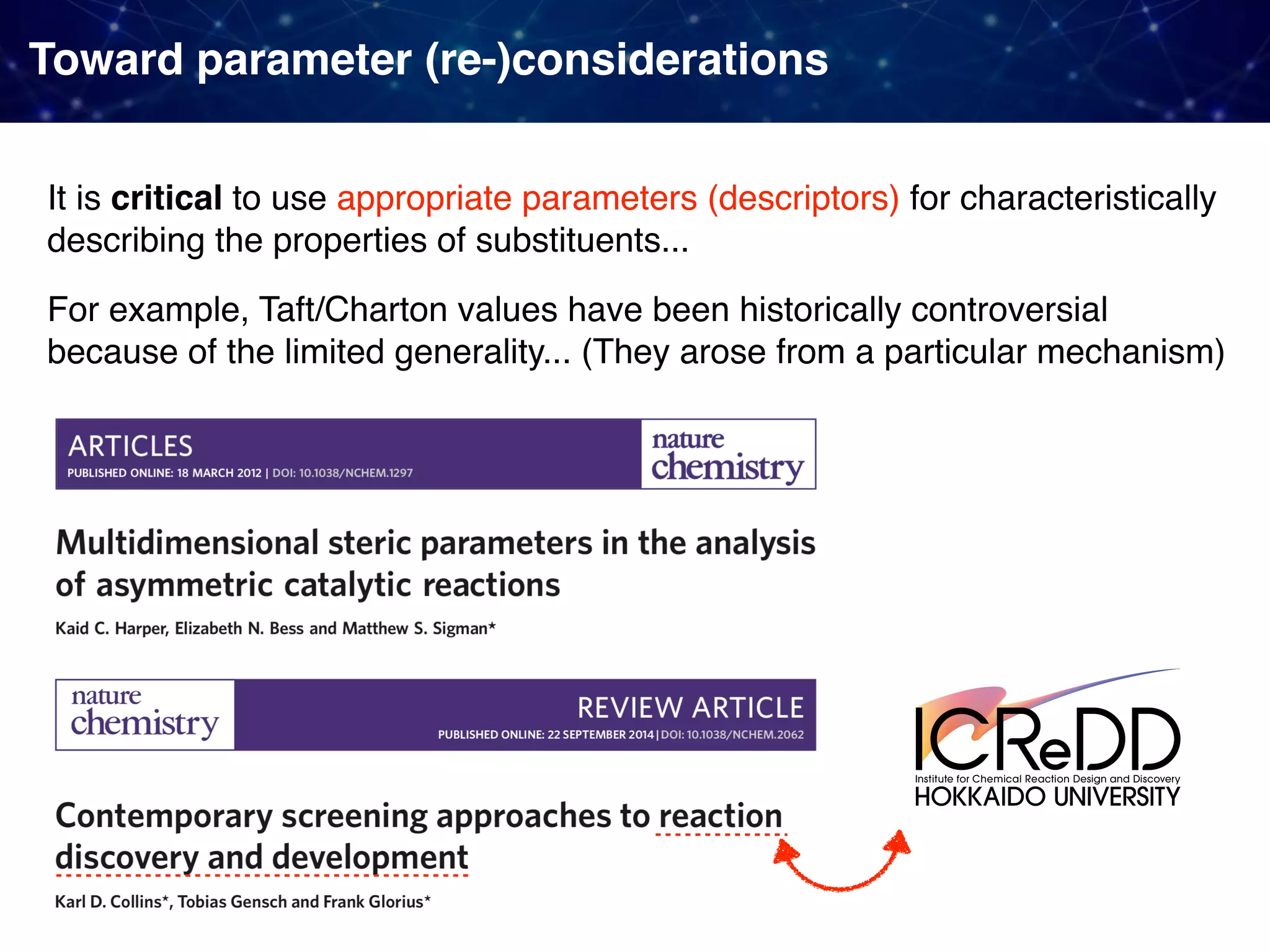
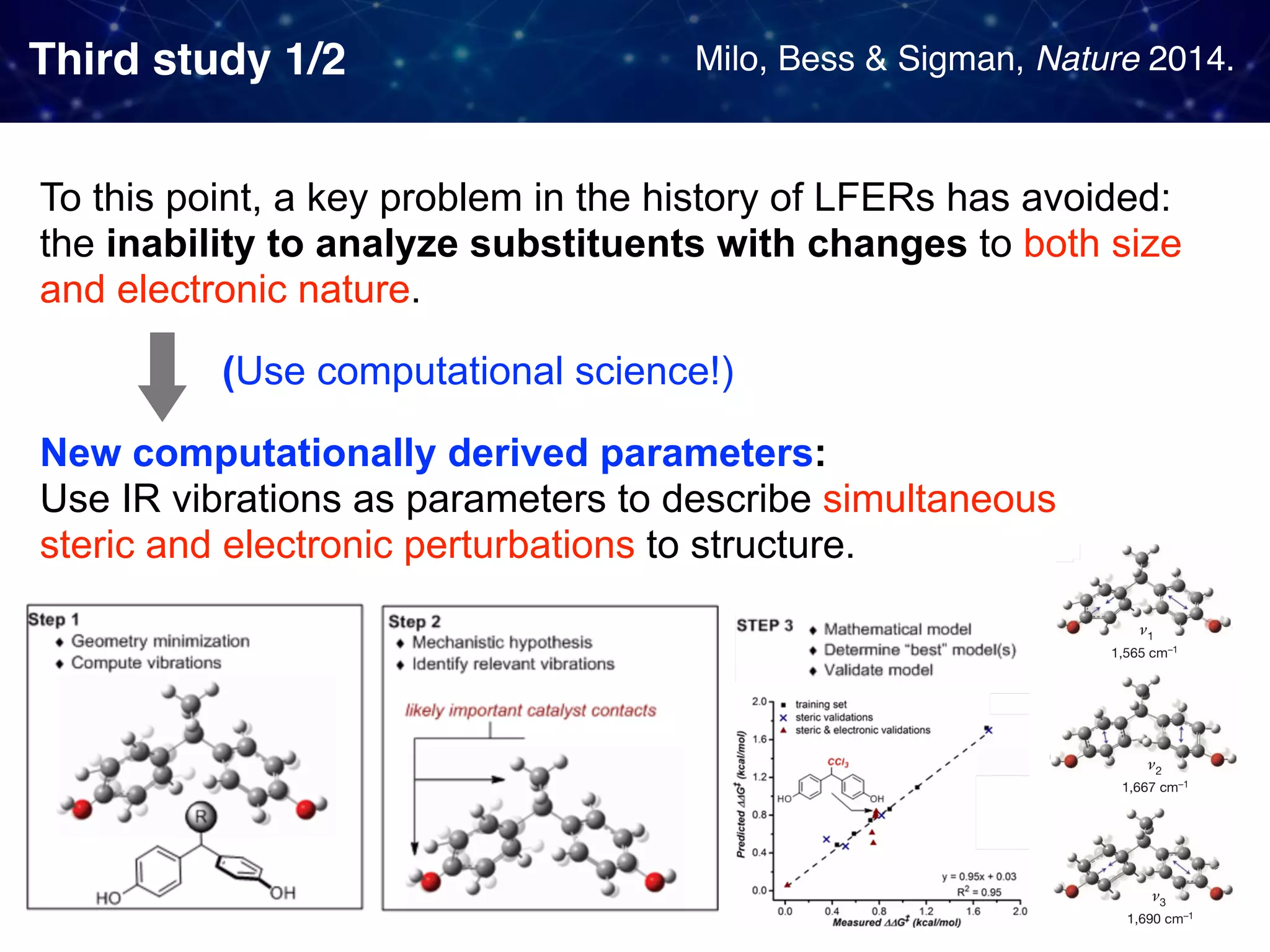
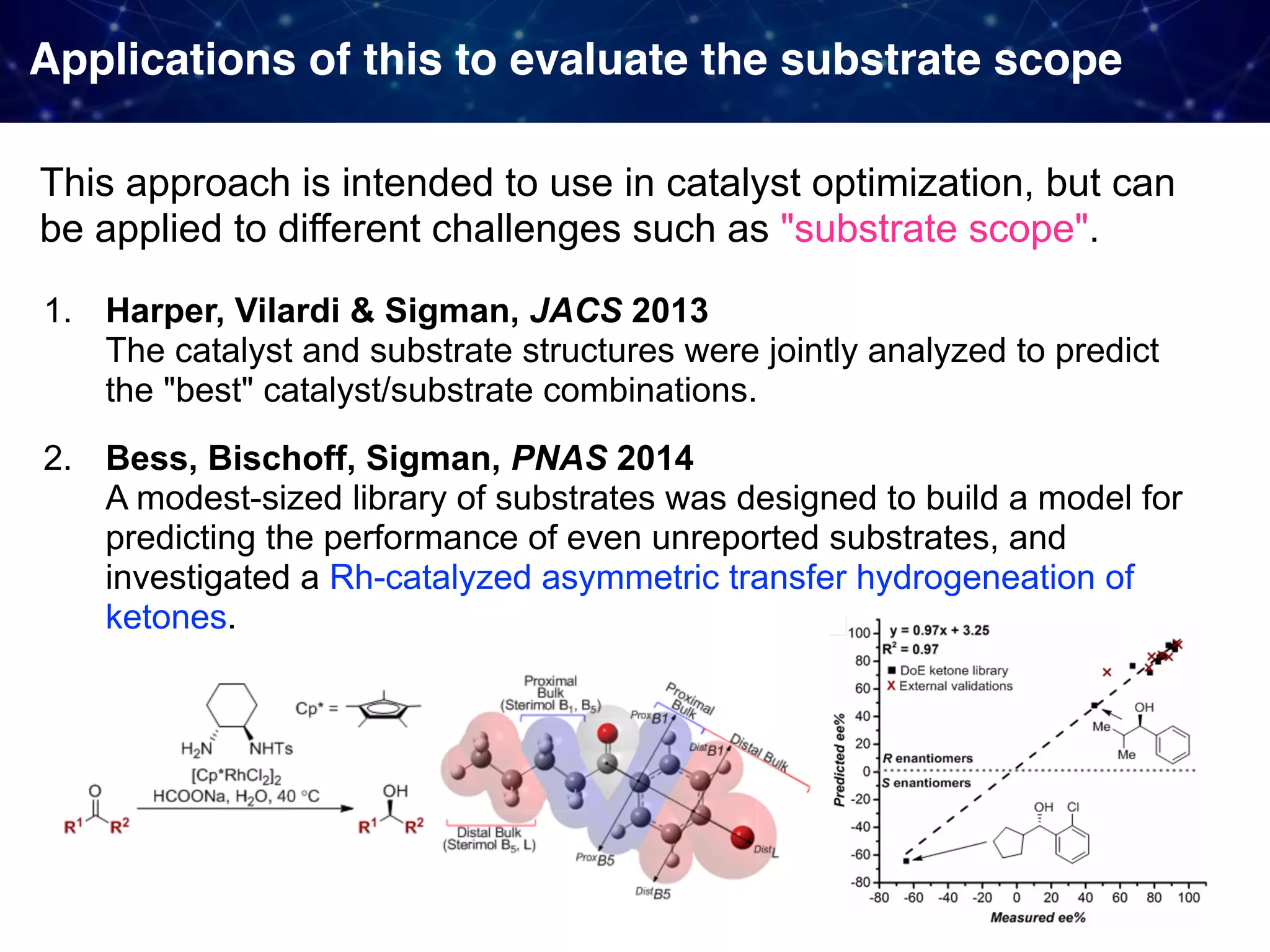
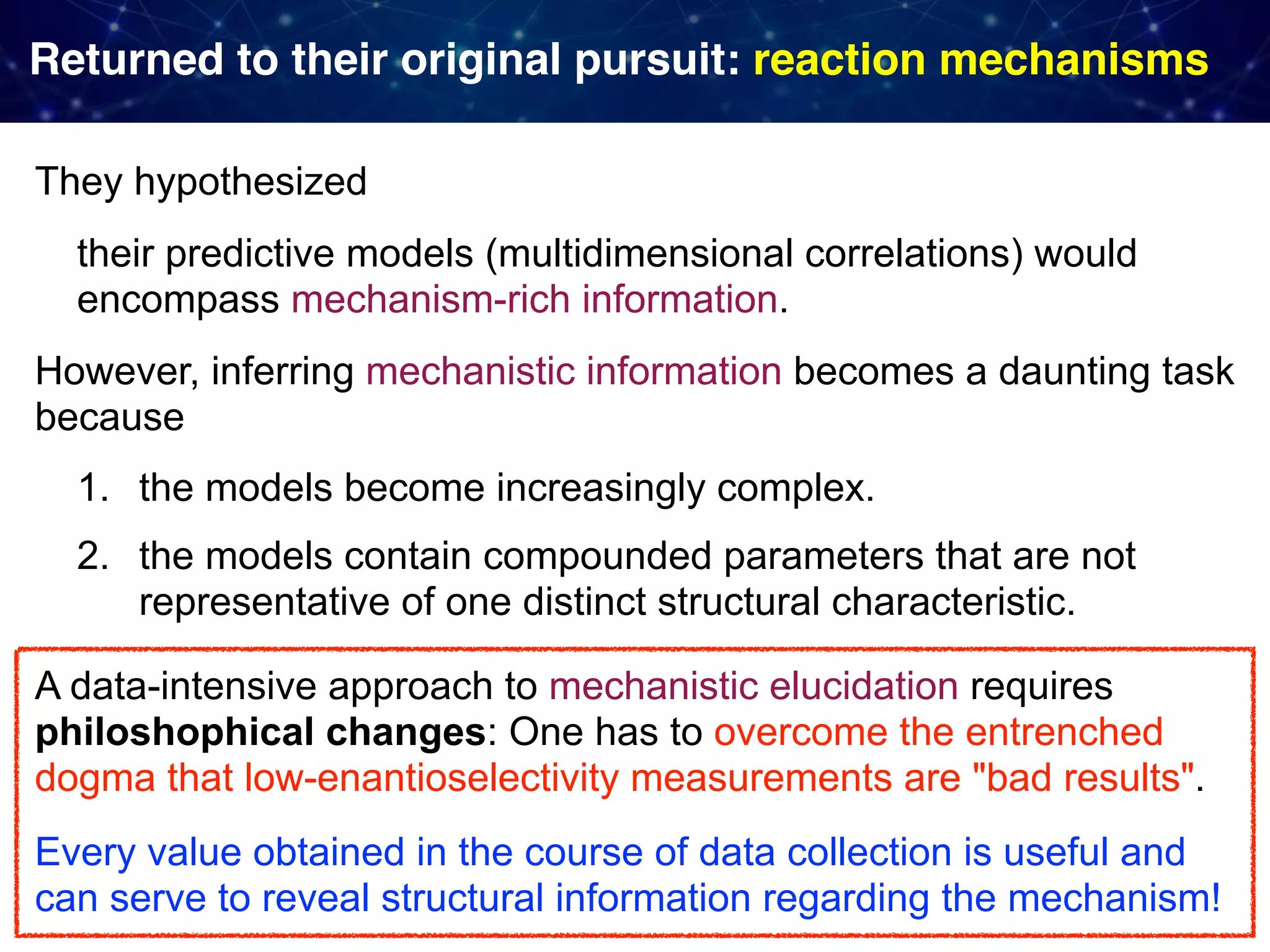
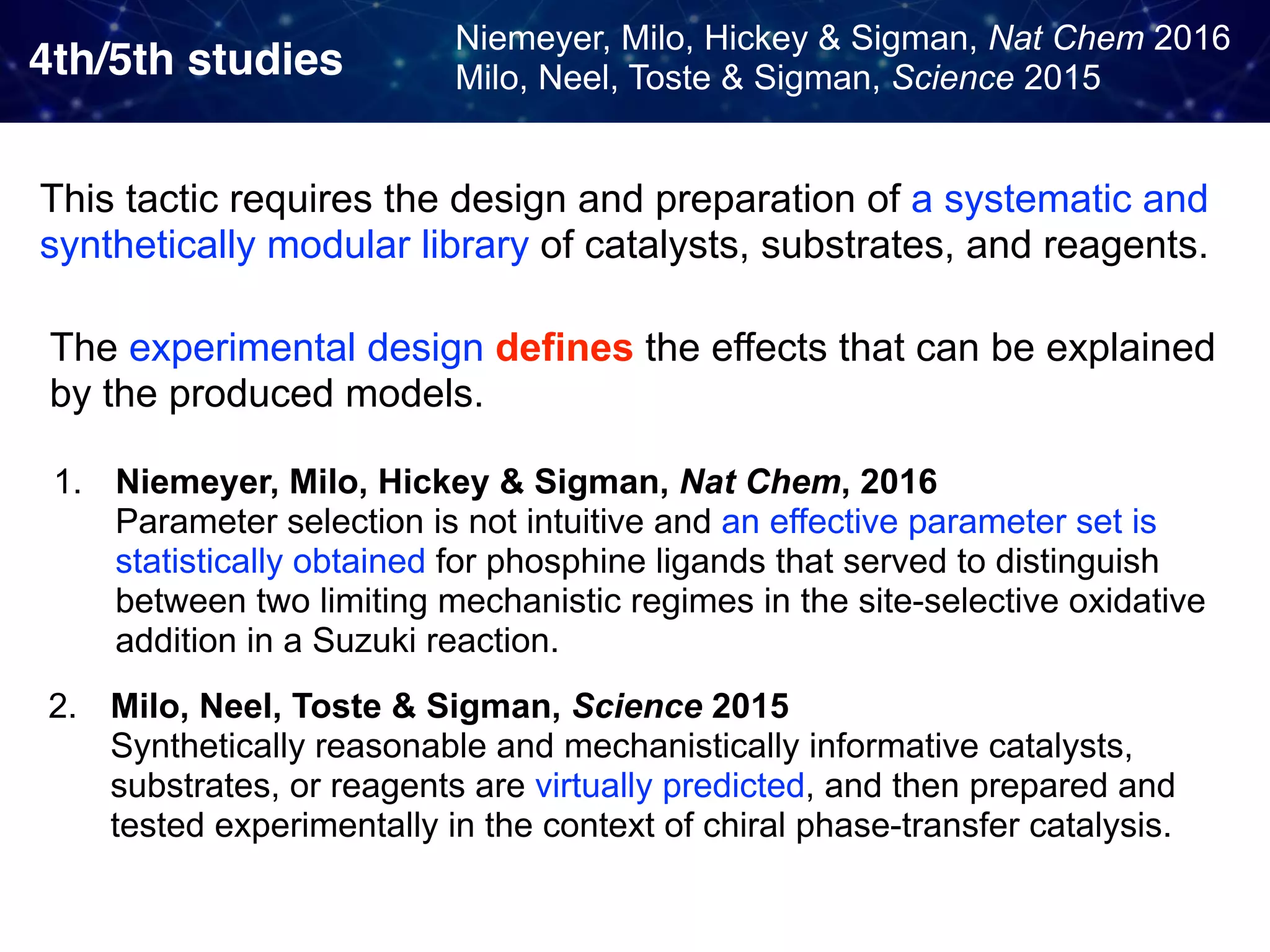
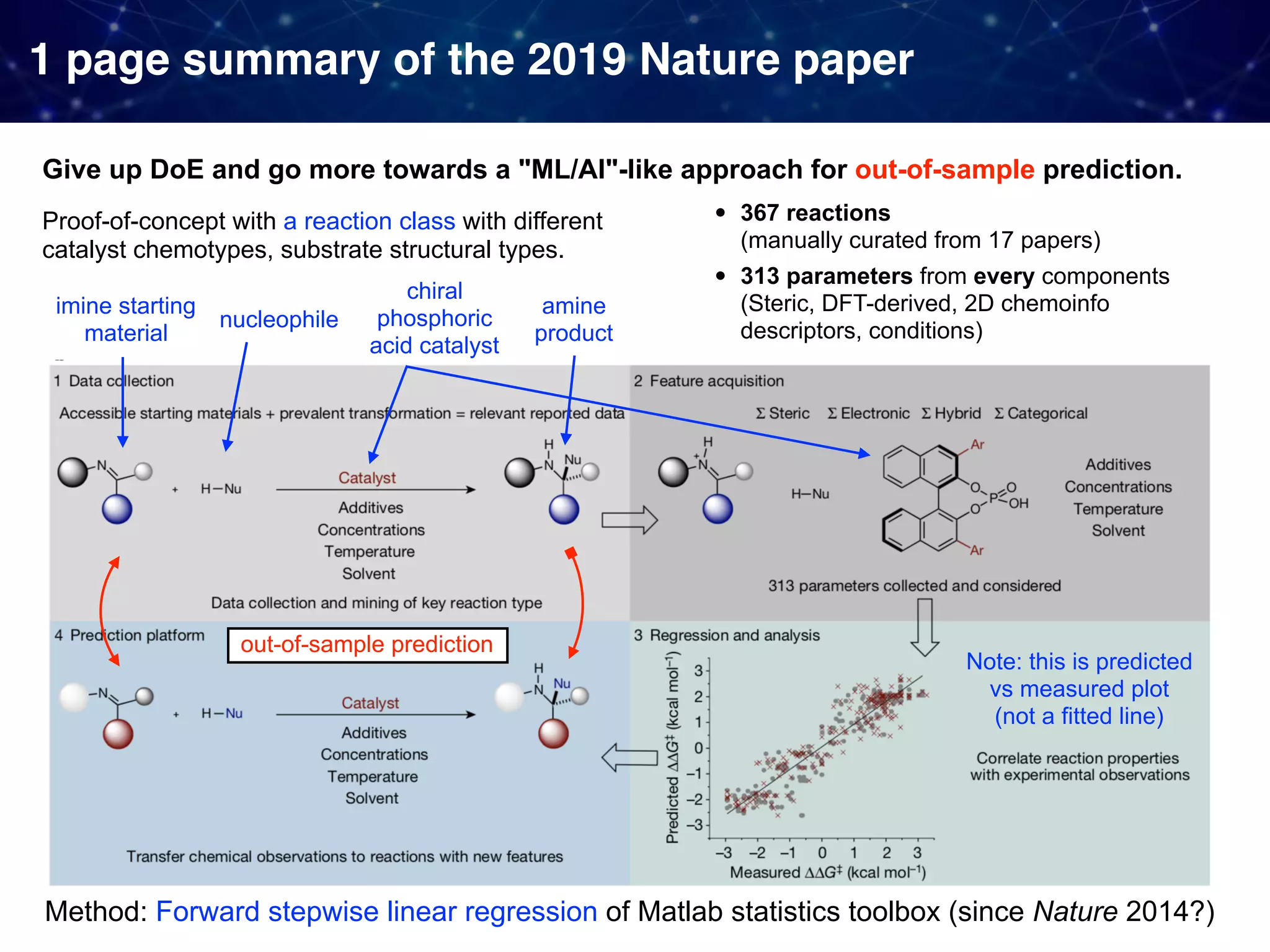
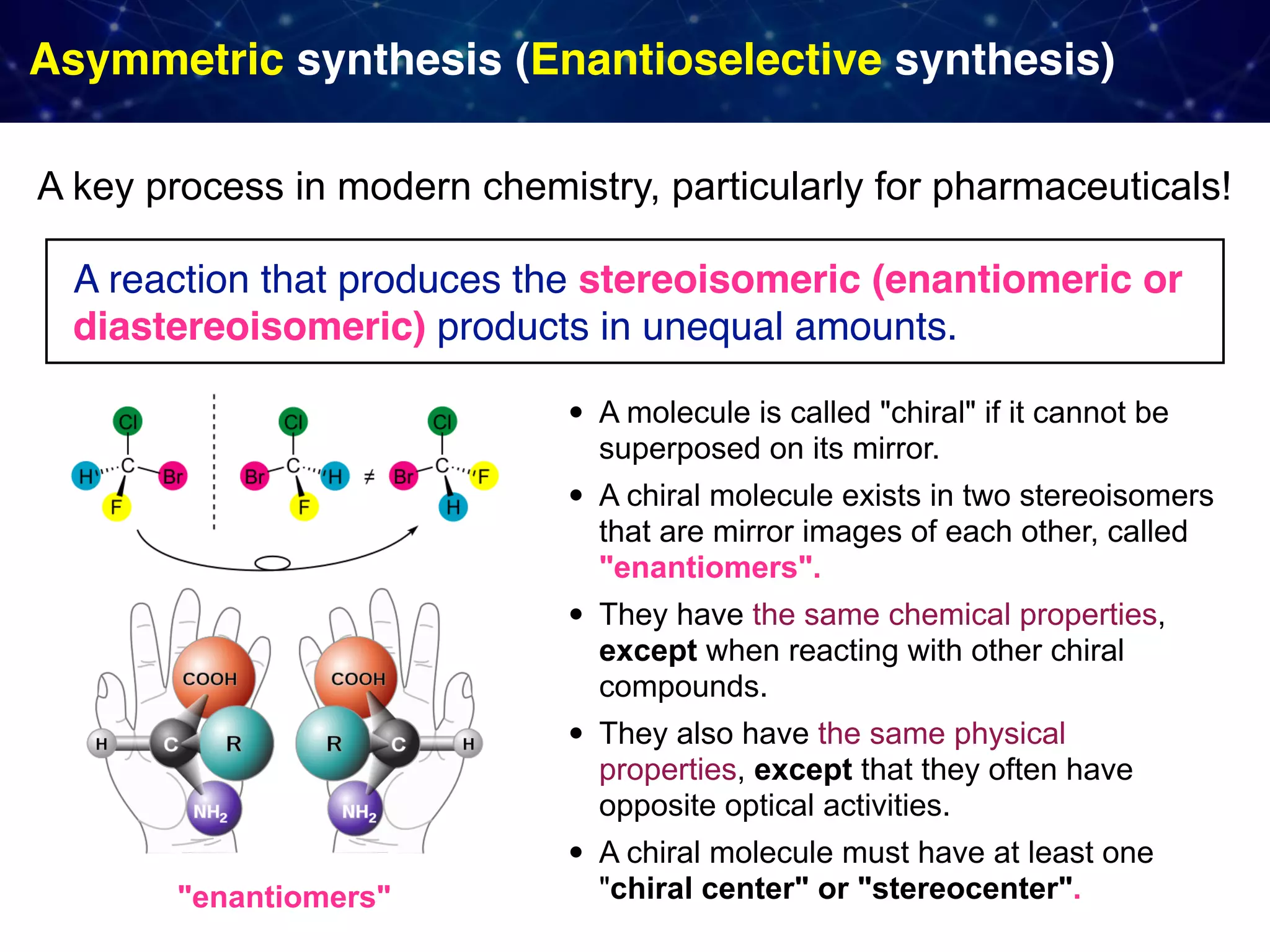
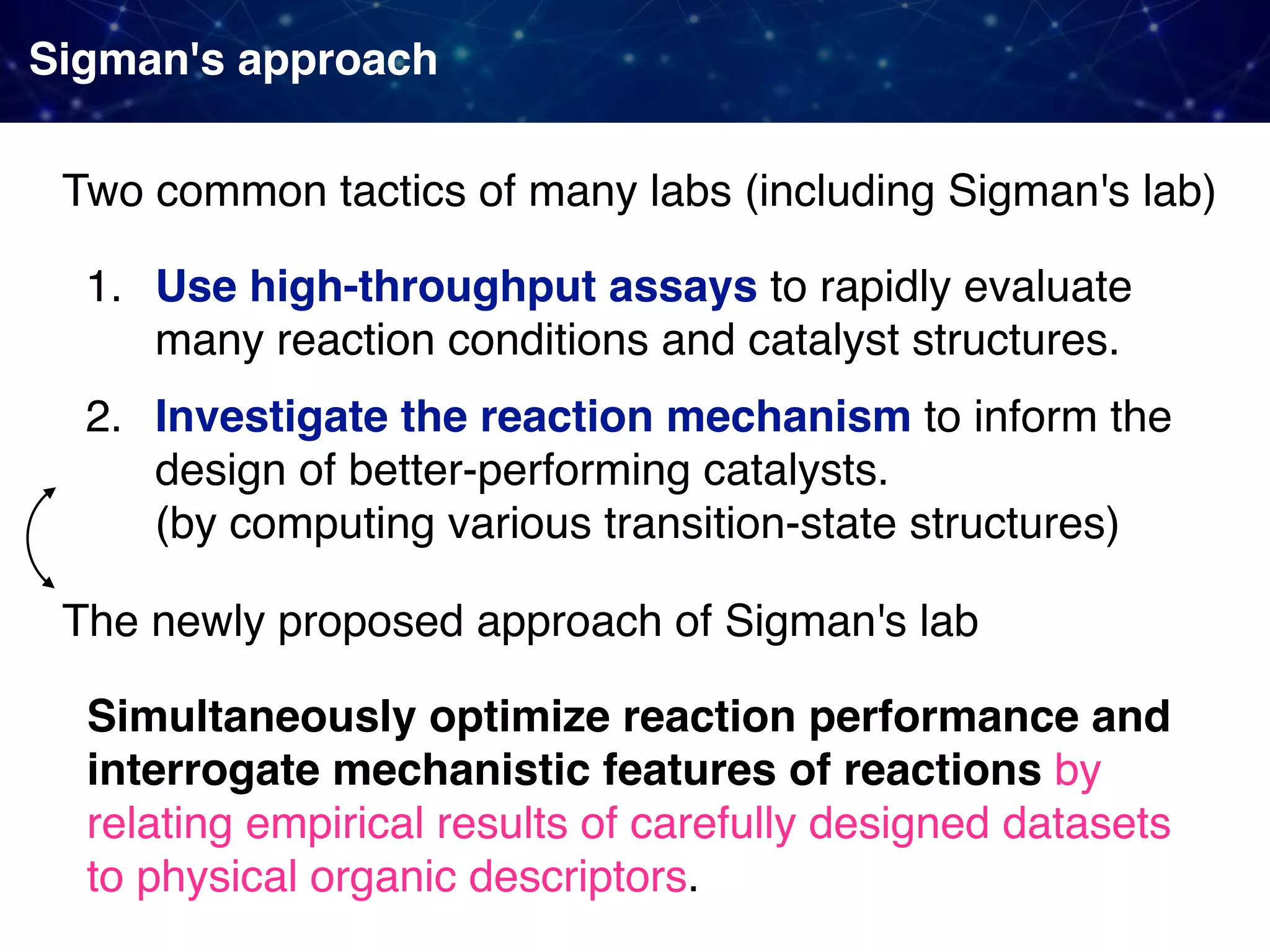
The document discusses strategies proposed by Matthew S. Sigman's lab for utilizing data to design and optimize asymmetric catalysis, focusing on the integration of chemoinformatics, statistical analysis, and high-throughput screening to identify effective catalysts. Key themes include the importance of understanding reaction mechanisms, applying design of experiments, and developing sophisticated descriptors to improve enantioselectivity. Several studies illustrate the use of empirical data to optimize catalyst structures and enhance reaction outcomes across various chemical contexts.



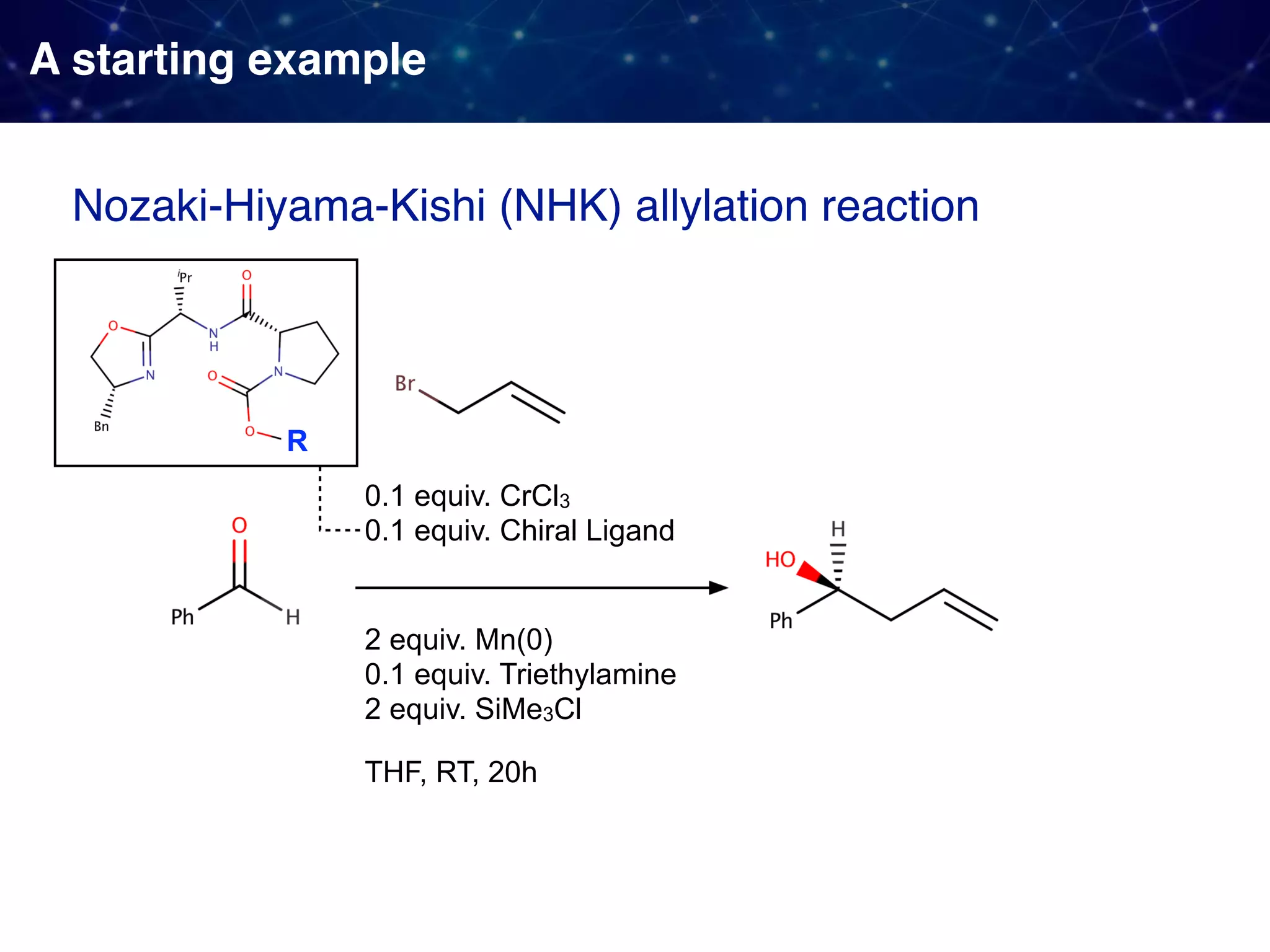
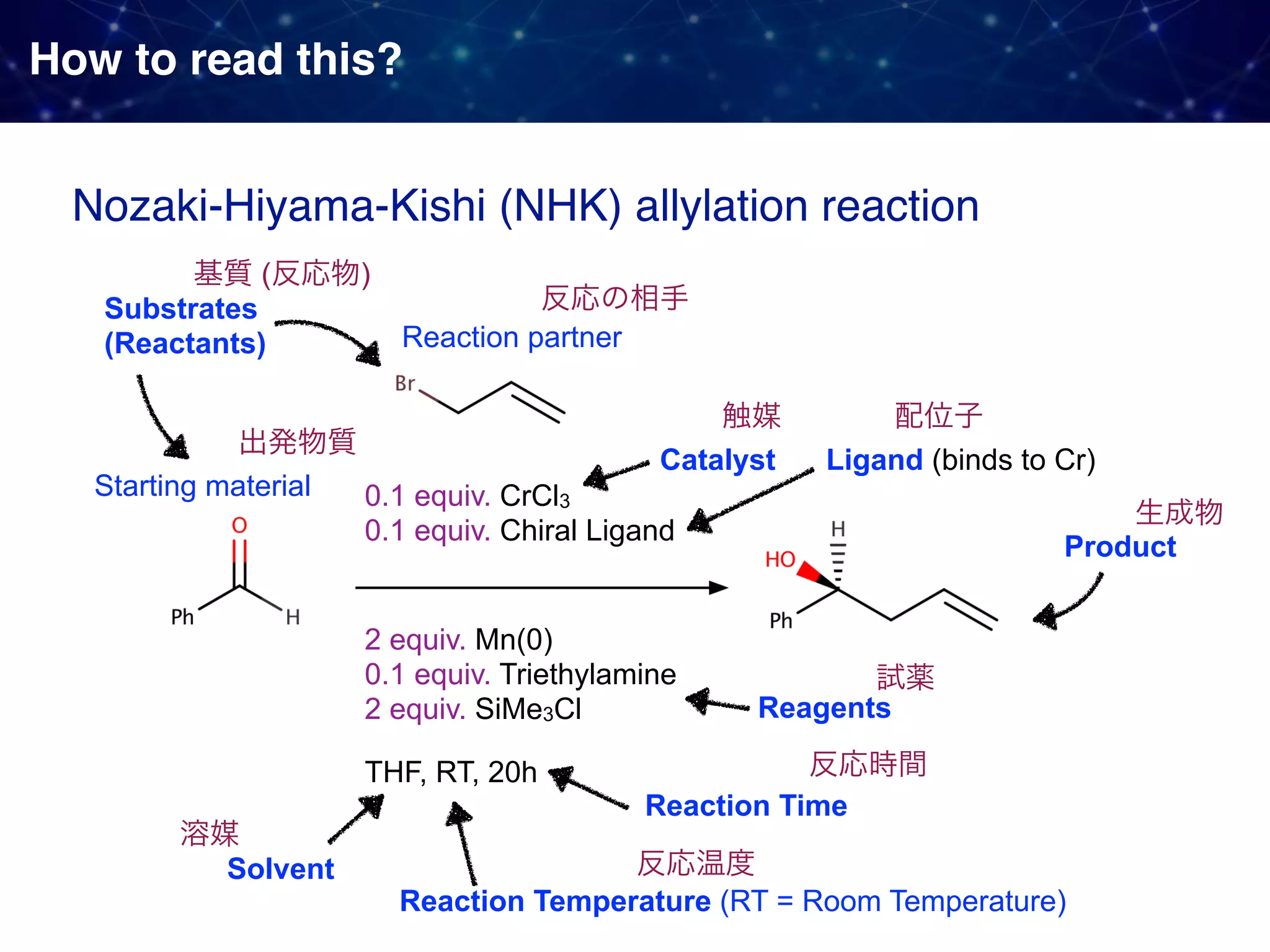
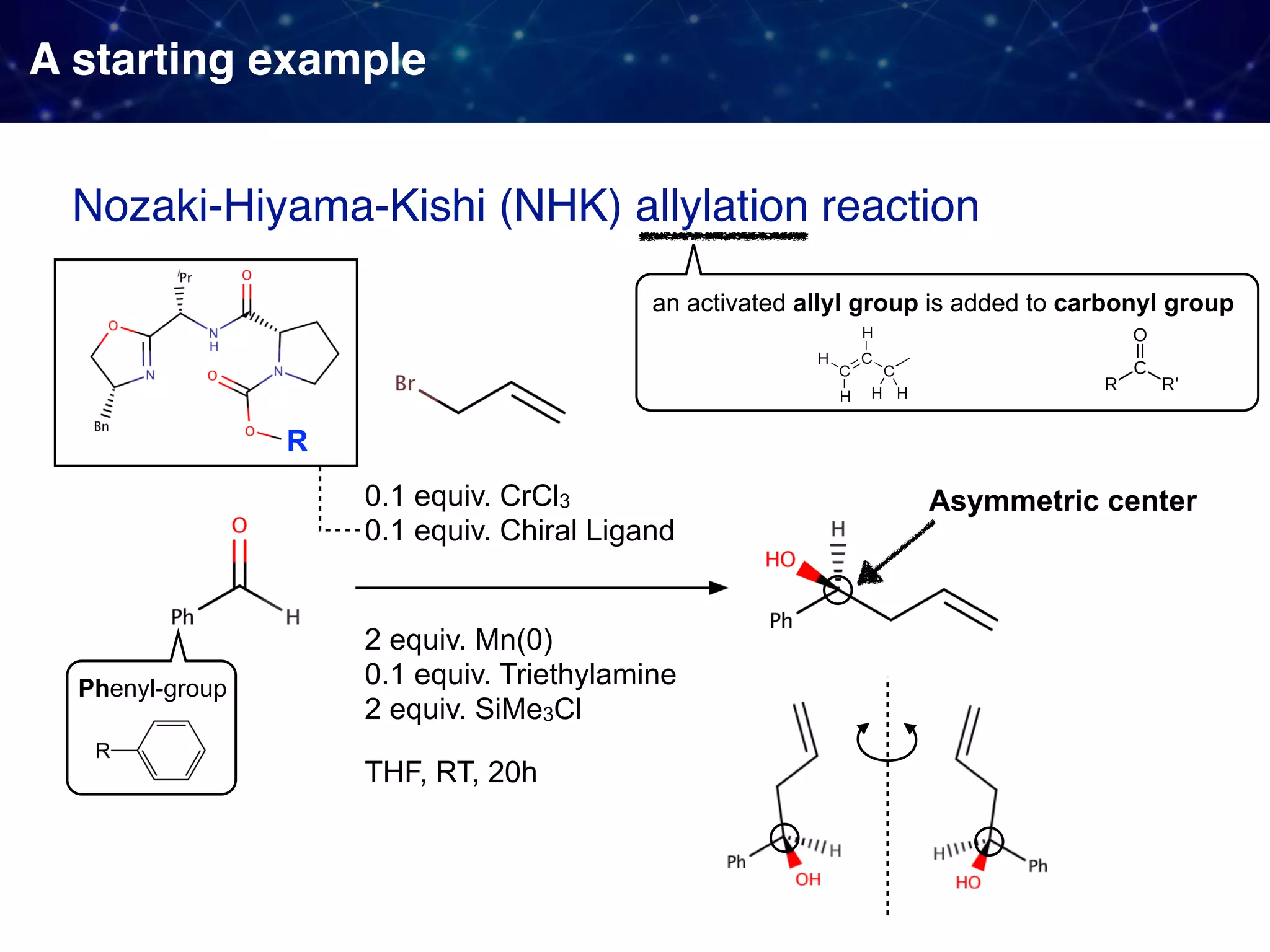
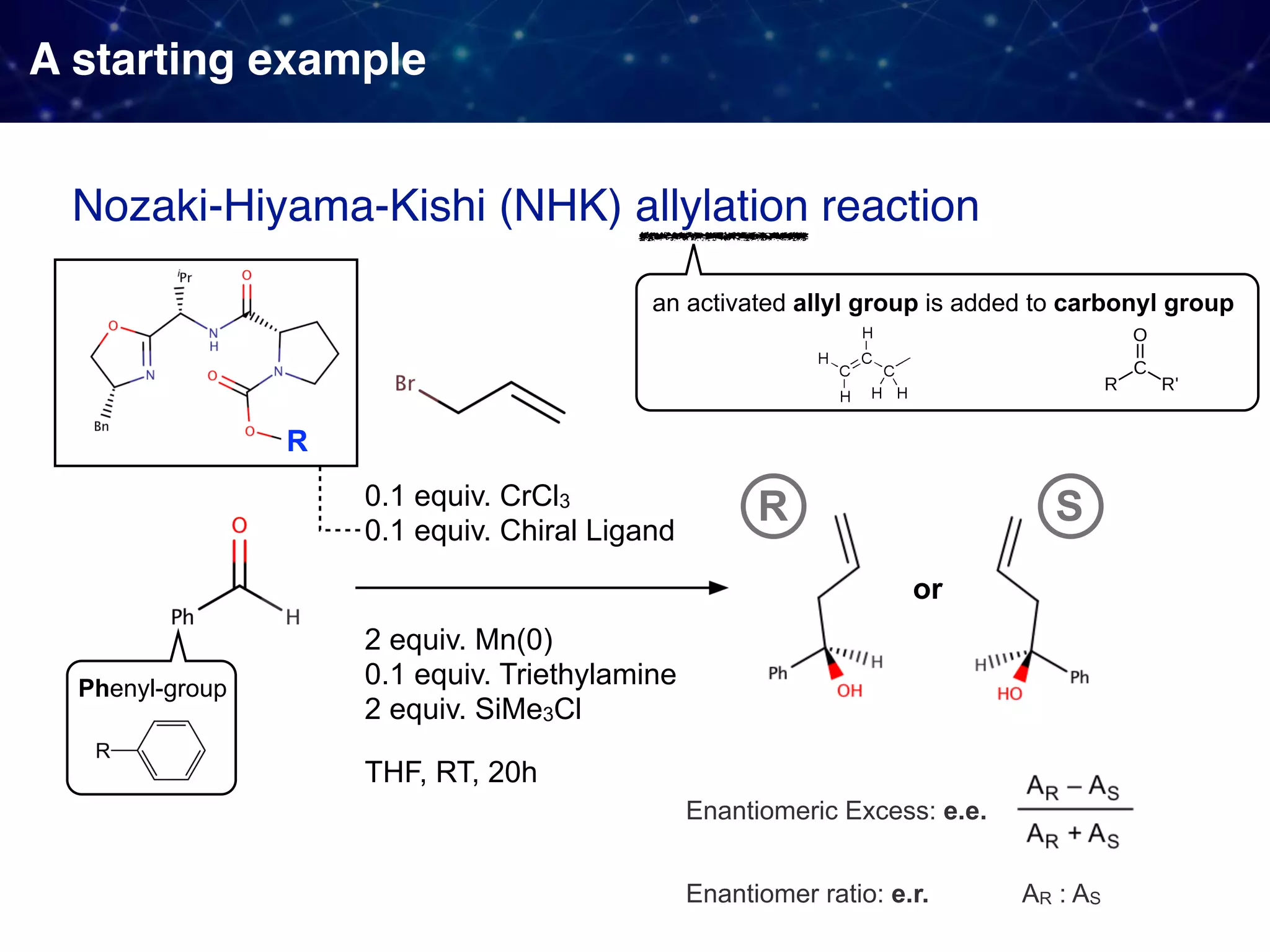
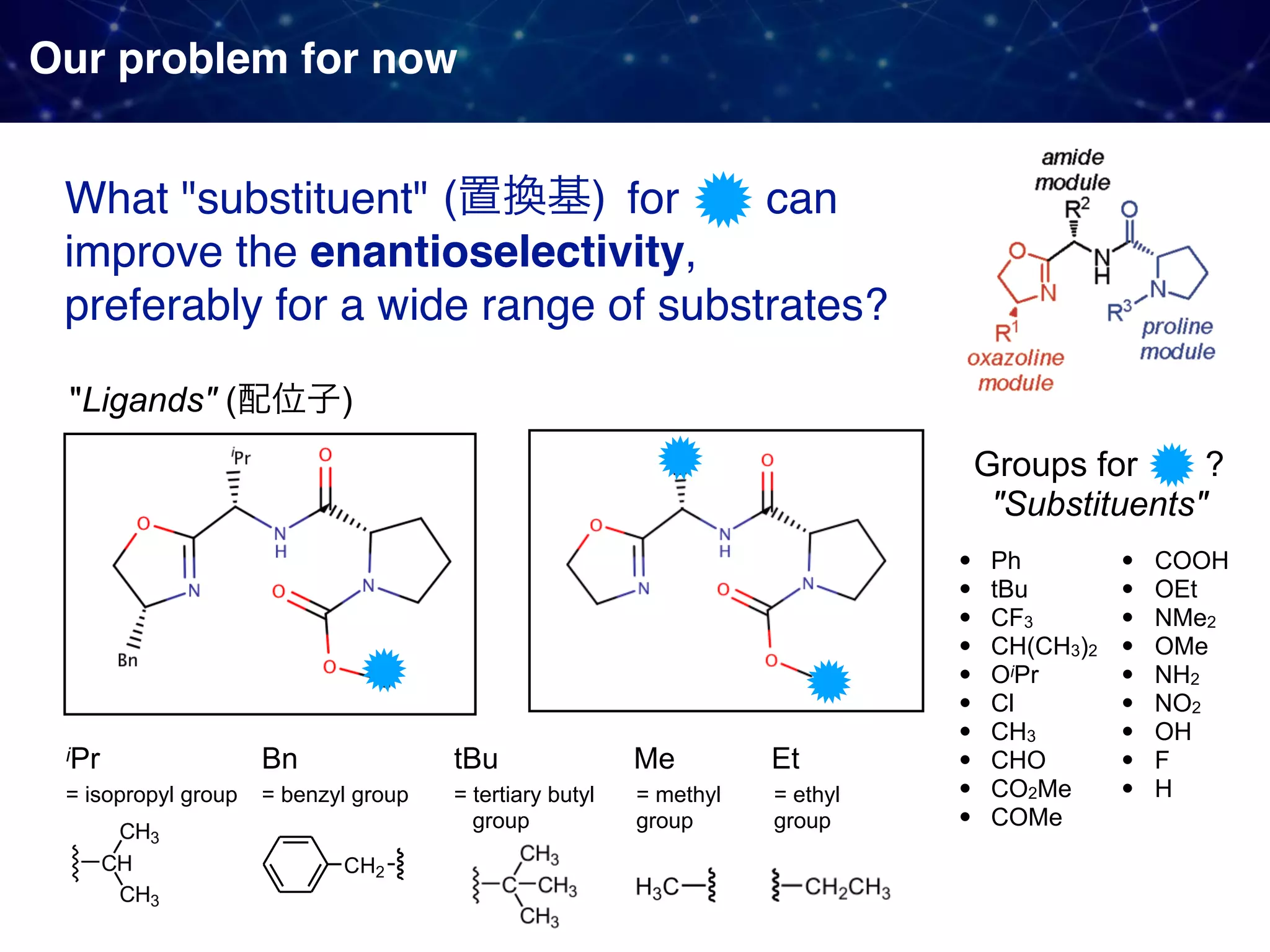
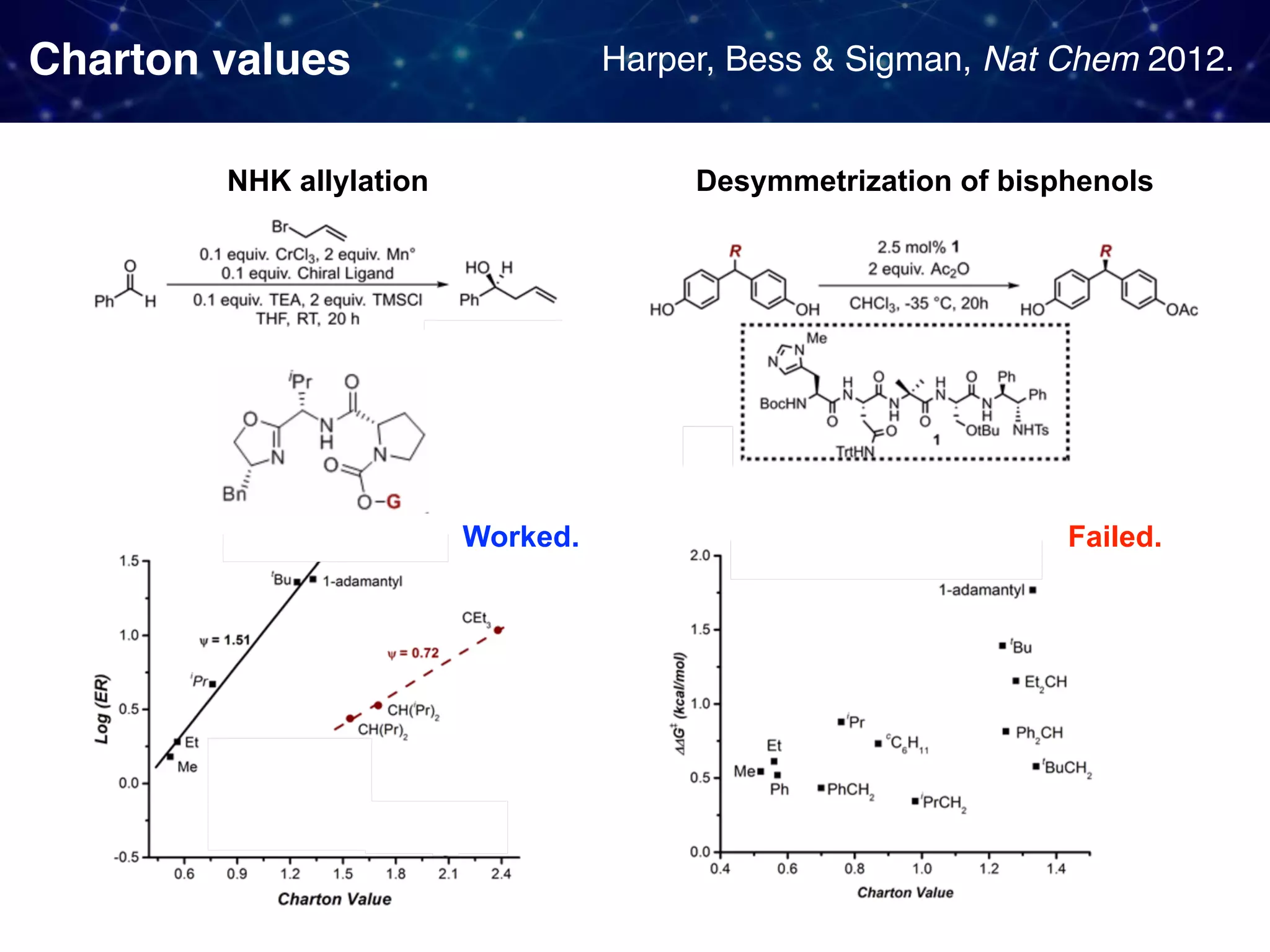
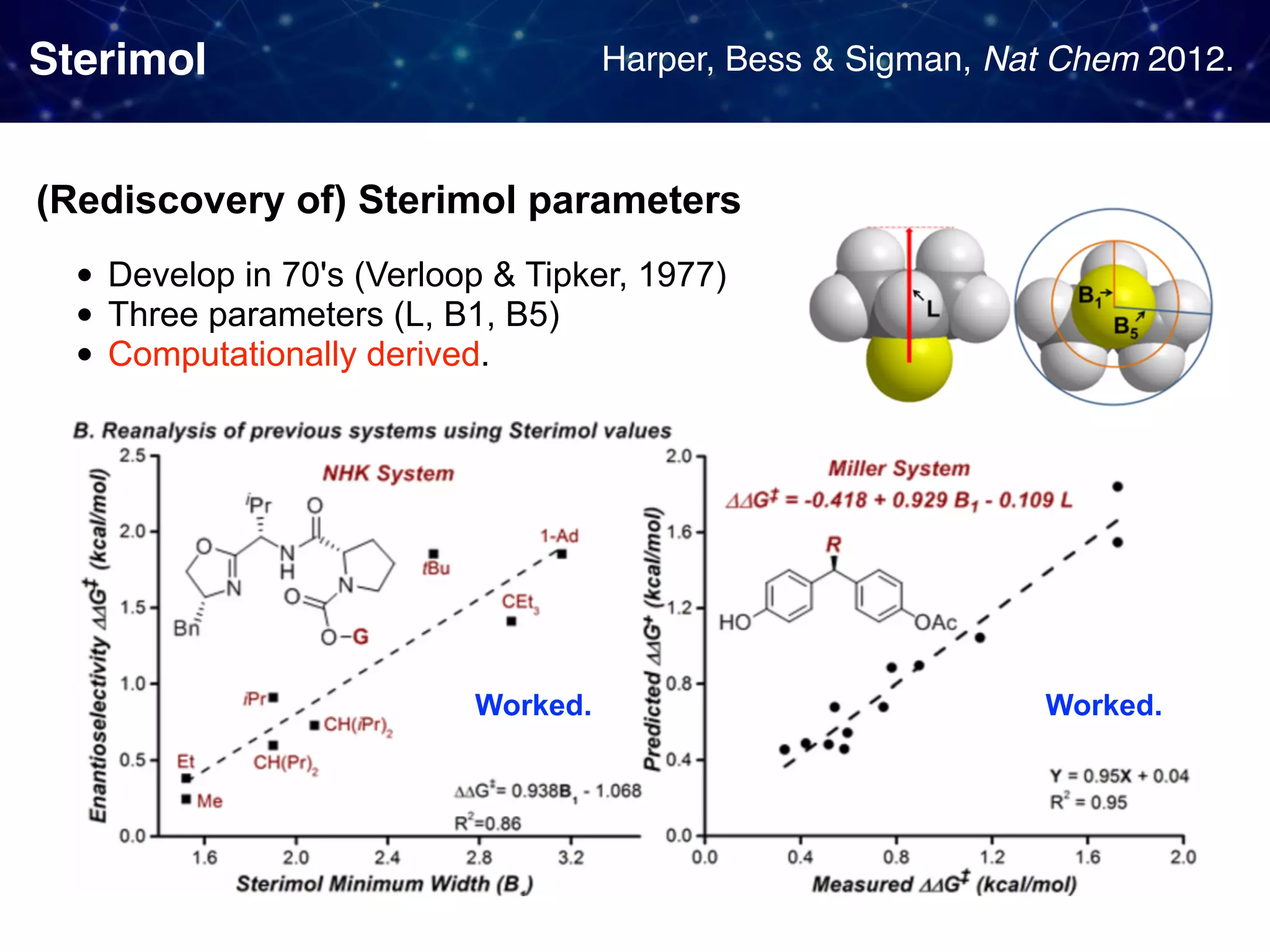
![Backstory before the new approach Sigman & Miller, J. Org. Chem. 2009, 74, 20, 7633-7643 They observed 1. Linear Free Energy Relationship (LFER):
the enantioselectivity and the size of the proline substituent G (measured by Taft/Charton steric parameters) were positively correlated. 2. A break in the LFER when larger G groups were employed. [ ]‡ means
a transition state unclear multistep reactions](https://image.slidesharecdn.com/slide20200427final-200428015742/75/How-to-use-data-to-design-and-optimize-reaction-A-quick-introduction-to-work-from-Sigman-lab-12-2048.jpg)